Apyrase in horticultural crops: insights into growth, stress adaptation and quality regulation
- PMID: 40887633
- PMCID: PMC12400707
- DOI: 10.1186/s43897-025-00168-w
Apyrase in horticultural crops: insights into growth, stress adaptation and quality regulation
Abstract
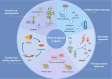
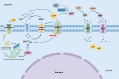
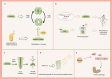
Apyrases are a kind of nucleoside triphosphate diphosphohydrolases that catalyze the removal of the terminal phosphate group from nucleoside triphosphate (NTP) or nucleoside diphosphate (NDP). They also function either intracellularly or extracellularly in mediating the NTP/NDP homeostasis critical for plant growth, development, senescence, stress response and adaptation. Initial studies elucidated the biochemistry, structure and function of plant apyrases, while the recent progresses include the crystallography, newly discovered interaction partners and downstream targets for diverse apyrases. Furthermore, these apyrases play diverse roles in horticultural crops with the new recognition of extracellular ATP (eATP) receptors. This review summarized the types, structures, biochemical and physiological functions of plant apyrases and highlighted their roles in plant growth, development, biotic/abiotic stress responses and adaptation. The physiological activities among the apyrases, eATP with its receptor and eATP/iATP homeostasis, were reviewed. In particular, the quality formation / deterioration of postharvest horticultural crops caused by apyrases was emphasized. This paper reviewed the recent advances in the multiple roles of apyrases in horticultural crops and provided insights into the regulation of physiological activities by the enzyme from molecular network perspectives.
Keywords: Apyrase; Energy; Extracellular ATP; Horticultural crops; Physiological activities; Signal transduction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests. Prof. Yueming Jiang is a member of the Editorial Board for Molecular Horticulture. He was not involved in the journal’s review of, and decisions related to, this manuscript.
Figures






References
-
- Aghdam MS, Jannatizadeh A, Luo Z, Paliyath G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci Tech. 2018;76:67–81. - DOI
-
- Aghdam MS, Flores FB, Sedaghati B. Exogenous phytosulfokine α (PSKα) application delays senescence and relieves decay in strawberry fruit during cold storage by triggering extracellular ATP signaling and improving ROS scavenging system activity. Sci Hortic. 2021;279:109906. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
