Development of a Machine Learning Model Integrating Pathomics and Clinical Data to Predict Axillary Lymph Node Metastasis in Breast Cancer: A Two-Center Study
- PMID: 40887934
- PMCID: PMC12399835
- DOI: 10.1002/cnr2.70302
Development of a Machine Learning Model Integrating Pathomics and Clinical Data to Predict Axillary Lymph Node Metastasis in Breast Cancer: A Two-Center Study
Abstract
Background: Accurately assessing the status of axillary lymph nodes (ALNs) is essential for devising optimal surgical plans and making informed treatment decisions in breast cancer (BC) patients.
Aims: This study aims to develop an innovative nomogram based on pathomics to preoperatively predict ALN metastasis (ALNM) in BC.
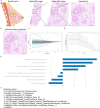
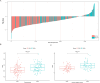
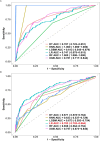
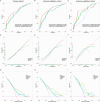
Methods and results: Our study performed a retrospective analysis on digital hematoxylin and eosin (H&E)-stained images obtained from 407 patients across two institutions who were allocated into a training cohort (TC; n = 203), an internal validation cohort (IVC; n = 136), and an external validation cohort (EVC; n = 68). Initially, the Mann-Whitney U-test and Spearman's rank correlation coefficient were utilized for feature selection, employing the least absolute shrinkage and selection operator (LASSO) regression for further refinement. For the evaluation of the predictive value of ALNM and other clinicopathological factors, we deployed both univariate (ULR) and multivariate (MLR) logistic regression analyses. Among the six machine learning (ML) algorithms, logistic regression, which demonstrated the highest area under the curve (AUC) value, was employed to establish the final nomogram model. The nomogram reliability and stability were assessed by analyzing the AUC of the receiver operating characteristic (ROC) curve, decision curve analysis (DCA), and calibration plots. MLR analysis demonstrated estrogen receptor (ER), human epidermal growth factor receptor 2 (HER2), tumor size, and pathomics features as independent ALNM predictors. The nomogram demonstrated that the AUC in the IVC (0.783) surpassed that of the Path-score model (0.698) (DeLong test, p = 0.008558). Similarly, in the EVC, the nomogram surpassed the clinical model regarding AUC (0.738 vs. 0.574; DeLong test, p = 0.00494). Additionally, DCA analysis indicated a net clinical benefit associated with the nomogram.
Conclusion: Our study demonstrates the effectiveness of pathomics features in predicting ALNM in BC patients. Furthermore, the pathomics-based nomogram offers a valuable tool for personalized treatment planning in this patient population.
Keywords: axillary lymph node metastasis; breast cancer; machine learning; nomogram model; pathomics.
© 2025 The Author(s). Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Developing and Evaluating a Nomogram Model Predicting Axillary Lymph Node Metastasis of Triple-Negative Breast Cancer Based on Multimodal Imaging Characteristics.Acad Radiol. 2025 Aug;32(8):4382-4394. doi: 10.1016/j.acra.2025.04.031. Epub 2025 May 15. Acad Radiol. 2025. PMID: 40379590
-
Identifying low-risk breast cancer patients for axillary biopsy exemption: a multimodal preoperative predictive model.Eur J Med Res. 2025 Jul 28;30(1):680. doi: 10.1186/s40001-025-02950-4. Eur J Med Res. 2025. PMID: 40717102 Free PMC article.
-
Establishment of Prediction Model of Axillary Lymph Node Metastasis Before Operation for Early-Stage Breast Cancer.Cancer Control. 2025 Jan-Dec;32:10732748251363328. doi: 10.1177/10732748251363328. Epub 2025 Jul 27. Cancer Control. 2025. PMID: 40717419 Free PMC article.
-
Establishment of an interpretable MRI radiomics-based machine learning model capable of predicting axillary lymph node metastasis in invasive breast cancer.Sci Rep. 2025 Jul 18;15(1):26030. doi: 10.1038/s41598-025-10818-0. Sci Rep. 2025. PMID: 40676103 Free PMC article.
-
Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2011 Jan;15(4):iii-iv, 1-134. doi: 10.3310/hta15040. Health Technol Assess. 2011. PMID: 21276372 Free PMC article.
References
-
- Tran H. T. and Jackson R. S., “ASO Author Reflections: Preoperative Nomogram Identifies Patients for Selective Omission of SLNB in Breast Cancer—To Promote Implementation of the Choosing Wisely Campaign Recommendations,” Annals of Surgical Oncology 27, no. 8 (2020): 2687–2688, 10.1245/s10434-020-08288-x. - DOI - PubMed
-
- Pilger T. L., Francisco D. F., and Candido Dos Reis F. J., “Effect of Sentinel Lymph Node Biopsy on Upper Limb Function in Women With Early Breast Cancer: A Systematic Review of Clinical Trials,” European Journal of Surgical Oncology 47, no. 7 (2021): 1497–1506, 10.1016/j.ejso.2021.01.024. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- 2024NSCQ-MSX1087/Natural Science Foundation of Chongqing
- 2023MSXM103/Chongqing Science and Health Joint Medical Research Project
- 2024166/Shapingba District Technology Innovation Project
- 2019NLTS005/Chongqing Municipal Health and Family Planning Commission
- CQYC20200303137-cstc2021ycjh-bgzxm0193/Talent Program of Chongqing
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

