Efficacy and Safety of Baxdrostat in Uncontrolled and Resistant Hypertension
- PMID: 40888730
- PMCID: PMC7618089
- DOI: 10.1056/NEJMoa2507109
Efficacy and Safety of Baxdrostat in Uncontrolled and Resistant Hypertension
Abstract
Background: Aldosterone dysregulation plays an important pathogenic role in hard-to-control hypertension. In several studies, baxdrostat, an aldosterone synthase inhibitor, reduced the seated systolic blood pressure of patients with uncontrolled or resistant hypertension.
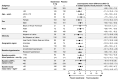
Methods: In this phase 3, multinational, double-blind, randomized, placebo-controlled trial, we recruited patients with a seated systolic blood pressure of between 140 mm Hg and less than 170 mm Hg despite the receipt of stable treatment with two antihypertensive medications (uncontrolled hypertension) or three or more such medications (resistant hypertension), including a diuretic. After a 2-week placebo run-in period, we randomly assigned patients with a seated systolic blood pressure of 135 mm Hg or more in a 1:1:1 ratio to receive baxdrostat at a dose of 1 mg, baxdrostat at a dose of 2 mg, or placebo once daily for 12 weeks. The primary end point was the change in seated systolic blood pressure from baseline to week 12.
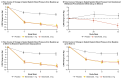
Results: A total of 796 patients underwent randomization and 794 received 1-mg baxdrostat (264 patients), 2-mg baxdrostat (266 patients), or placebo (264 patients) in addition to background therapy. At 12 weeks, the change from baseline in the least-squares mean seated systolic blood pressure was -14.5 mm Hg (95% confidence interval [CI], -16.5 to -12.5) with 1-mg baxdrostat, -15.7 mm Hg (95% CI, -17.6 to -13.7) with 2-mg baxdrostat, and -5.8 mm Hg (95% CI, -7.9 to -3.8) with placebo. The estimated difference from placebo (placebo-corrected difference) was -8.7 mm Hg (95% CI, -11.5 to -5.8) with 1-mg baxdrostat and -9.8 mm Hg (95% CI, -12.6 to -7.0) with 2-mg baxdrostat (P<0.001 for both comparisons). A potassium level of more than 6.0 mmol per liter was reported in 6 patients (2.3%) with 1-mg baxdrostat, in 8 patients (3.0%) with 2-mg baxdrostat, and in 1 patient (0.4%) with placebo.
Conclusions: Among patients with uncontrolled or resistant hypertension, the addition of baxdrostat to background therapy resulted in a significantly lower seated systolic blood pressure at 12 weeks than placebo. (Funded by AstraZeneca and others; BaxHTN ClinicalTrials.gov number, NCT06034743.).
Copyright © 2025 Massachusetts Medical Society.
Figures
References
-
- Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. - PubMed
-
- Monticone S, Sconfienza E, D’Ascenzo F, et al. Renal damage in primary aldosteronism. J Hypertens. 2020;38:3–12. - PubMed
-
- Williams B, MacDonald TM, Morant SV, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6:464–75. doi: 10.1016/S2213-8587(18)30071-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
