Real-world experience with the pentaspline pulsed field ablation system: one-year outcomes of the FARADISE registry
- PMID: 40888735
- PMCID: PMC12400809
- DOI: 10.1093/europace/euaf182
Real-world experience with the pentaspline pulsed field ablation system: one-year outcomes of the FARADISE registry
Abstract
Aims: Clinical studies with protocol-mandated workflow and monitoring have analysed performance of pulsed field ablation (PFA) for treating atrial fibrillation (AF). The FARADISE registry captures global use of the pentaspline PFA catheter in real-world clinical practice with a follow-up of 3 years.
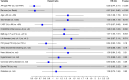
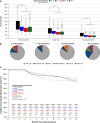
Methods and results: FARADISE is a prospective, non-randomized, multi-national registry (NCT05501873) that enrolled subjects clinically indicated for ablation using the pentaspline PFA catheter per medical judgement and hospital standard-of-care. Procedural characteristics, safety, and clinical effectiveness up to 12-months were collected. In total, 1158 AF patients received PFA across 48 centres in 21 countries (64 ± 11 years, 33% female, 90% de novo, 65% paroxysmal AF). Pulmonary vein isolation (PVI)-only procedures were performed in 80.8% of paroxysmal vs. 57.5% for non-paroxysmal patients (P < 0.01). Median procedure, left atrial dwell, and fluoroscopy times were 51[40-70], 31[24-41], and 12[8-17] min, respectively. The rate of early onset serious adverse events was 1.5% and did not differ by ablation strategy or AF indication. At 1-year, clinical effectiveness was 80.8% for paroxysmal AF and 67.7% for non-paroxysmal AF, with no difference within indication by lesion set (paroxysmal: 81.2% PVI-only vs. 79.0% PVI+, P = 0.65; non-paroxysmal: 67.5% PVI-only vs. 67.7% PVI+, P = 0.79). Acute results reinforce a short procedural learning curve with no difference in 1-year effectiveness by operator experience.
Conclusion: The FARADISE registry provides a snapshot of real-world clinical use of the pentaspline PFA catheter. Acute results demonstrate favourable procedural and safety outcomes regardless of AF indication. One-year outcomes are encouraging, with no differences seen within indication based on ablation strategy.
Keywords: Atrial fibrillation; Learning curve; Pulsed field ablation; Real-world.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: L.V.A.B.—Consultant and speaker for Boston Scientific, Medtronic, ZOLL, Biosense Webster. Grants from Boston Scientific and Medtronic. All fees and funding go to the Cardiology Department and hospital. G.S.—personal fees from Abbott, Bayer, Boston Scientific, Biosense Webster and Luma Vision, not related to the present study. A.d.R.- Consultant and speaker for Boston Scientific, Medtronic, Abbott, Biosense Webster. N.S.—Consultant for Boston Scientific, Abbott, Johnson and Johnson, not related to the present study. S.W.—consultant and study funding from Boston Scientific and Abbott, speaking honoraria for Boston Scientific, Abbott, Johnson & Johnson, Bristol-Myers Squibb, and Medtronic. H.H.—received consulting and speaking fees from Abbott and Boston Scientific and have served on the Patient Safety Advisory Board of Boston Scientific. F.S.—received consultancy fees From Boston Scientific. G.A.—Consultant and speaker fees for Boston Scientific, Medtronic and Abbot. A.L.—consultant and speaker for Boston Scientific, Pfizer, Bristol-Myers Squibb, Daiichi Sankyo and Abiomed. P.L.—reports receiving lecture honoraria from Medtronic, Boston Scientific, and Pfizer Pharma, as well as educational support from Boston Scientific and Johnson & Johnson. P.L. also serves as a consultant and speaker for Boston Scientific, Medtronic, and Biosense Webster. L.E.—discloses consultant fees, speaking honoraria, and travel expenses from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boehringer, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Sanofi Aventis. Research has been supported by German Research Foundation (DFG) and German Heart Foundation outside the submitted work. A.O.—consulting fees Johnson & Johnson, Abbott, Medtronic. J.O.—Speaker honoraria for Boston Scientific and Abbot. N.C., M.J., J.D.R., E.M.A., and B.S.S.—Employees of Boston Scientific. J.V.—Research grants for the cardiology department from Boston Scientific, Biosense Webster, Medtronic, Biotronik, Abbott. All remaining authors have declared no conflicts of interest.
Figures




Comment in
-
Another day in FARADISE? Lessons learned from a real-world global registry of pulsed field ablation with the pentaspline catheter for atrial fibrillation ablation.Europace. 2025 Sep 1;27(9):euaf193. doi: 10.1093/europace/euaf193. Europace. 2025. PMID: 40888740 Free PMC article. No abstract available.
References
-
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. - PubMed
-
- Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2024;149:e1–156. - PMC - PubMed
-
- Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2023;388:105–16. - PubMed
-
- Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. - PubMed
-
- Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

