Content Validity of the Spinocerebellar Ataxia Composite Score as a Measure of Disease Progression in Patients with Spinocerebellar Ataxia
- PMID: 40888983
- PMCID: PMC12401751
- DOI: 10.1007/s12311-025-01896-x
Content Validity of the Spinocerebellar Ataxia Composite Score as a Measure of Disease Progression in Patients with Spinocerebellar Ataxia
Abstract
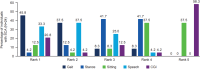
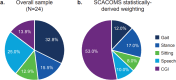
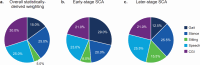
Spinocerebellar ataxia (SCA) composite score (SCACOMS) is a statistically-derived composite measure comprising weighted items that are sensitive to change during early-stage disease. SCACOMS items and weights include the functional Scale for the Assessment and Rating of Ataxia Gait (12%), Stance (17%), Sitting (8%), and Speech (10%) items, and the Clinician Global Impression of Change (CGI) (53%). The content validity of SCACOMS is yet to be established. Semi-structured qualitative interviews were conducted with individuals with SCA (N = 24) and healthcare professionals (HCPs) who treat SCA (N = 2) to evaluate the relevance and weighting of SCACOMS items for assessment of SCA progression. Interviews were audio recorded, transcribed, coded, and analyzed using ATLAS.Ti v23 software, following established methods. SCACOMS items measured all sign and symptom concepts reported by > 50.0% of individuals with SCA, including difficulties with speech (79.2%), balance (75.0%), and gait (66.7%). Of SCACOMS items, individuals with SCA ranked Gait as most important for assessing disease progression (45.8%) and CGI as least important (58.3%). When weighting SCACOMS items, individuals with SCA assigned the highest weight to Gait (mean [standard deviation], 32.8% [13.24]) and the lowest weight to Sitting (12.9% [7.98]). HCPs varied the item weights dependent on SCA severity. Agreement with the statistically-derived weighting of SCACOMS varied, with 41.6% of individuals with SCA indicating that CGI weighting was too high. Overall, all participants indicated that SCACOMS could detect meaningful changes and/or disease stabilization. This study supports the content validity of SCACOMS in SCA; however, SCACOMS item weights may warrant adjustment.
Keywords: Clinical outcomes assessment; Content validity; Spinocerebellar ataxia; Spinocerebellar ataxia composite score.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Patient Consent and Ethical Approval: All eligible individuals with SCA and the HCPs provided informed consent to participate in the interviews and could withdraw at any time. The study was conducted in accordance with the 1964 Declaration of Helsinki. Competing Interests: Maggie Heinrich, Katja Rudell, Naomi Suminski, Rinchen Doma, Kavita Jarodia, Mahak Jain, and Chris Buckley are employees of Parexel International and have been compensated as consultants by Biohaven Pharmaceuticals Inc. Linda Abetz-Webb received consultancy fees from Parexel International for this study. Jeremy Schmahmann has received compensation as a consultant for Biohaven Pharmaceuticals Inc., is site Principal Investigator for the Biohaven clinical trials NCT03701399 and NCT02960893, and has received an unrestricted grant from Biohaven for an imaging biomarker study in multiple system atrophy. Susan Perlman has been a member of an advisory board or consultant for Biogen, Biohaven Pharmaceuticals, Inc, Erydel, PTC, Quince Therapeutics, Reata Pharmaceuticals, and Steminent. Liana Rosenthal is an Associate Professor in the Department of Neurology at the Johns Hopkins University School of Medicine. She engaged in this research as a private consultant or advisor and not in her capacity as a Johns Hopkins faculty member, and was compensated. Liana Rosenthal also receives: research funding support from the NINDS, the Daniel B. and Florence E. Green Family Foundation, and the Macks Family Foundation; programmatic support from the Gordon and Marilyn Macklin Foundation; and salary support for her role as site Principal Investigator for research studies with Biohaven Pharmaceuticals, Pfizer, and EIP Pharma and for serving on the Clinical Events Committee for a research study with Functional Neuromodulation. Liana Rosenthal also serves on steering committees for the Parkinson Study Group’s research study with both UCB and Bial Pharmaceuticals, and on advisory boards for Reata pharmaceuticals, Biogen pharmaceuticals, Biohaven Pharmaceuticals, Merck Healthcare KGaA, and was a one-time consultant for UCB pharmaceuticals.Michele Potashman, Melissa Wolfe-Beiner, Vlad Coric, and Gilbert L’Italien are employed by and hold stock/stock options in Biohaven Pharmaceuticals Inc.
Figures
References
-
- Klockgether T, Lüdtke R, Kramer B, Abele M, Bürk K, Schöls L, et al. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121(Pt 4):589–600. 10.1093/brain/121.4.589. - PubMed
-
- Perlman S. Hereditary ataxia overview. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews. Seattle, USA: University of Washington; 1993. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

