The quality of patient decision aids for lung cancer screening: Results from an environmental scan
- PMID: 40889250
- PMCID: PMC12401189
- DOI: 10.1002/cncr.70008
The quality of patient decision aids for lung cancer screening: Results from an environmental scan
Abstract
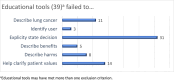
Shared decision making is recommended for lung cancer screening (LCS) by professional organizations and payers. Patient decision aids can be used to support shared decision making, but they need to meet quality standards to minimize the potential for biased and poorly informed patient decisions. After the updated LCS recommendation from the US Preventive Services Task Force in 2021, the authors conducted an environmental scan of public-facing patient educational materials and evaluated them against criteria from the International Patient Decision Aid Standards for high-quality patient decision aids. The Google site search function was used to search websites from National Cancer Institute-funded cancer centers, professional societies, patient advocacy groups, cancer coalitions, and private organizations for educational materials on LCS. A general web search using Google, Google Scholar, and select databases was also conducted. Considerations unique to the LCS context (e.g., the importance of annual screening and smoking cessation) were documented. The search identified 96 educational materials that included information about both benefits and harms of LCS. Of these, 39 did not meet qualifying criteria for decision aids, with failure to explicitly identify LCS as a decision being the primary reason for exclusion. Only 10 of the remaining decision aids met quality criteria from the International Patient Decision Aid Standards. These aids emphasized that LCS should be performed annually, most avoided stigmatizing language, and several included personalization features using prediction models. Clinicians and patients can be confident in using these high-quality aids to complement the process of shared decision making for LCS. Validated aids in languages other than English and Spanish are needed.
Keywords: cancer early detection; decision aids; lung neoplasms, patient education, shared decision making.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Lauren S. Rosenthal and Robert A. Smith report that the American Cancer Society receives unrestricted educational funding by AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi‐Sankyo, Foundation Medicine, Genentech, Gilead, Guardant Health, Johnson & Johnson, Merck, Novartis, Novocure, Regeneron, Roche, Sanofi‐Genzyme, and Takeda in the form of sponsorships to support the American Cancer Society National Lung Cancer Roundtable. The remaining authors disclosed no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Decision aids for people facing health treatment or screening decisions.Cochrane Database Syst Rev. 2011 Oct 5;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2014 Jan 28;(1):CD001431. doi: 10.1002/14651858.CD001431.pub4. PMID: 21975733 Updated.
-
Decision aids for people facing health treatment or screening decisions.Cochrane Database Syst Rev. 2014 Jan 28;(1):CD001431. doi: 10.1002/14651858.CD001431.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Apr 12;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. PMID: 24470076 Updated.
-
Supporting community translation of lung cancer screening: A web-based decision aid to support informed decision making.Transl Behav Med. 2025 Jan 16;15(1):ibae073. doi: 10.1093/tbm/ibae073. Transl Behav Med. 2025. PMID: 39817729 Clinical Trial.
-
Decision aids for people facing health treatment or screening decisions.Cochrane Database Syst Rev. 2009 Jul 8;(3):CD001431. doi: 10.1002/14651858.CD001431.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2011 Oct 05;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. PMID: 19588325 Updated.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous