Coagulation factor XII haploinsufficiency is protective against venous thromboembolism in a population-scale multidimensional analysis
- PMID: 40890099
- PMCID: PMC12402198
- DOI: 10.1038/s41467-025-62789-5
Coagulation factor XII haploinsufficiency is protective against venous thromboembolism in a population-scale multidimensional analysis
Abstract
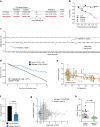
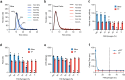
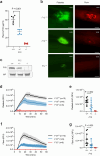
Coagulation factor XII has been identified as a potential drug target that could prevent thrombosis without increasing the risk of bleeding. However, human data to support the development of factor XII-directed therapeutics are lacking. To assess the role of factor XII in venous thromboembolism, we examine genetic variation in the coding region of the F12 locus across 703,745 participants in the UK Biobank and NIH All of Us biorepositories. We find that heterozygous carriers of nonsense, frameshift, and essential splice site variants in F12 are protected against venous thromboembolism without an increased risk of bleeding or infection. We also show that F12 variant carriers generally experience a quantitative (type I) defect in circulating factor XII levels, though a subset of participants was also identified with possible qualitative (type II) deficiency. In vitro plasma-based thrombin generation is reduced at factor XII concentrations reflective of those seen in F12 variant carriers. We also show that F12 heterozygous mice are protected against venous thromboembolism and display an intermediate phenotype between wild-type and F12-null animals. We conclude that heterozygous loss of F12 represents a haploinsufficient state characterized by protection against venous thromboembolism and that therapeutically inhibiting factor XII is likely to be safe and effective.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Agnelli, G. et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med.382, 1599–1607 (2020). - PubMed
-
- Granger, C. B. et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med.365, 981–992 (2011). - PubMed
-
- Connolly, S. J. et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med.364, 806–817 (2011). - PubMed
-
- Investigators, E.-P. et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med.366, 1287–1297 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

