Leveraging quantitative systems pharmacology modeling for elranatamab regimen optimization in relapsed or refractory multiple myeloma
- PMID: 40890126
- PMCID: PMC12402305
- DOI: 10.1038/s41540-025-00585-z
Leveraging quantitative systems pharmacology modeling for elranatamab regimen optimization in relapsed or refractory multiple myeloma
Abstract
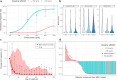
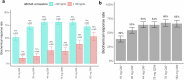
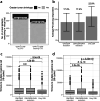
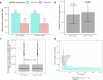
Elranatamab, an approved bispecific antibody (BsAb) for relapsed/refractory multiple myeloma, forms an immune synapse between the T-cell CD3 marker and B-cell maturation antigen (BCMA) on myeloma cells. Circulating soluble BCMA (sBCMA) is associated with disease burden and may reduce drug exposure, impacting efficacy. A quantitative systems pharmacology model that captures elranatamab's mechanism of action and disease dynamics was developed and calibrated to clinical datasets. Simulations explored model uncertainty and inter-patient variability with respect to biological, pharmacologic, and tumor-related components to inform clinical dose-response relationships and evaluate the effect of baseline sBCMA levels on dose and regimen. Model simulations supported 76 mg weekly as the optimal regimen, including in patients with high sBCMA. A left shift in the dose-response curve among virtual responders supported maintenance of efficacy with less frequent dosing. This work exemplifies how mechanistic models may support BsAb dose and regimen justification within the framework of model-informed drug development.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors were employees of Pfizer when the work took place.
Figures


References
-
- Furukawa, Y. & Kikuchi, J. Molecular basis of clonal evolution in multiple myeloma. Int. J. Hematol.111, 496–511 (2020). - PubMed
-
- Kumar, S. K. et al. Multiple myeloma. Nat. Rev. Dis. Prim.3, 17046 (2017). - PubMed
-
- Moreau, P. et al. Newly diagnosed myeloma in 2020. American Society of Clinical Oncology Educational Book, p. e144-e158 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

