REV-ERBα regulates brain NAD+ levels and tauopathy via an NFIL3-CD38 axis
- PMID: 40890338
- PMCID: PMC12532575
- DOI: 10.1038/s43587-025-00950-x
REV-ERBα regulates brain NAD+ levels and tauopathy via an NFIL3-CD38 axis
Abstract
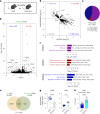
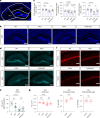
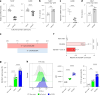
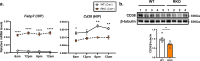
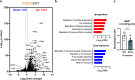
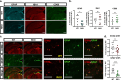
Nicotinamide adenine dinucleotide (NAD+) is a critical metabolic co-enzyme implicated in brain aging, and augmenting NAD+ levels in the aging brain is an attractive therapeutic strategy for neurodegeneration. However, the molecular mechanisms of brain NAD+ regulation are incompletely understood. In cardiac tissue, the circadian nuclear receptor REV-ERBα has been shown to regulate NAD+ via control of the NAD+-producing enzyme NAMPT. Here we show that REV-ERBα controls brain NAD+ levels through a distinct pathway involving NFIL3-dependent suppression of the NAD+-consuming enzyme CD38, particularly in astrocytes. REV-ERBα deletion does not affect NAMPT expression in the brain and has an opposite effect on NAD+ levels as in the heart. Astrocytic REV-ERBα deletion augments brain NAD+ and prevents tauopathy in P301S mice. Our data reveal that REV-ERBα regulates NAD+ in a tissue-specific manner via opposing regulation of NAMPT versus CD38 and define an astrocyte REV-ERBα-NFIL3-CD38 pathway controlling brain NAD+ metabolism and neurodegeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




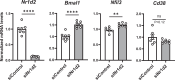






References
MeSH terms
Substances
Grants and funding
- RS-2019-NR040055/National Research Foundation of Korea (NRF)
- R01AG063743/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- RF1AG061776/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R21 AG089851/AG/NIA NIH HHS/United States
- R35NS097273/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01DK45586/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P01NS084974-01/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RF1AG062077/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- RF1AG062171/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

