Ligand triplet energy escape in lanthanide complexes for developing luminescent molecular thermometers
- PMID: 40890469
- PMCID: PMC12402069
- DOI: 10.1038/s42004-025-01673-1
Ligand triplet energy escape in lanthanide complexes for developing luminescent molecular thermometers
Abstract
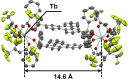
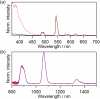
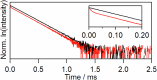
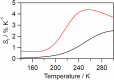
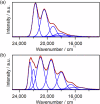
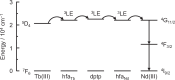
Luminescent lanthanide complexes can exhibit temperature-sensitive metal-centered emission due to energy transfer quenching from the lanthanide to the ligand triplet states, which have been promising application in emission lifetime-based thermometers. However, the long-lived ligand triplet state limits the temperature sensitivity of lanthanide emission. This study demonstrates an enhancement in the temperature sensitivity of Tb(III) emission by introducing an energy escape pathway from the ligand triplet state. A dinuclear Tb(III)-Nd(III) complex containing hexafluoroacetylacetonate (hfa) and triphenylene bridging ligands was prepared, which exhibits temperature-dependent energy transfer from the Tb(III)-emitting state to the hfa triplet state. The triplet level of the hfa ligand is similar to that of the triphenylene ligand, inducing effective energy transfer from hfa to Nd(III) via the triphenylene ligands. This energy transfer pathway provides a short-lived excited state of hfa ligands, resulting in the highest temperature sensitivity (4.4% K-1) among emission lifetime-based thermometers of lanthanide complexes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Schäferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed.51, 3532–3554 (2012). - PubMed
-
- Zhang, K. Y. et al. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev.118, 1770–1839 (2018). - PubMed
-
- Zhou, J., del Rosal, B., Jaque, D., Uchiyama, S. & Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods17, 967–980 (2020). - PubMed
-
- Wang, X. D. & Wolfbeis, O. S. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem. Soc. Rev.43, 3666–3761 (2014). - PubMed
Grants and funding
- JP24K21774/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP23H04863/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20H02748/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJTR23T5/MEXT | JST | Adaptable and Seamless Technology Transfer Program through Target-Driven R and D (Adaptable and Seamless Technology Transfer Program through Target-Driven R&D)
LinkOut - more resources
Full Text Sources

