Esketamine alleviates depressive-like behavior in mice via modulation of the microbiota-gut-brain axis and amino acid metabolism
- PMID: 40890568
- PMCID: PMC12400578
- DOI: 10.1186/s12866-025-04181-3
Esketamine alleviates depressive-like behavior in mice via modulation of the microbiota-gut-brain axis and amino acid metabolism
Abstract
Background: The microbiota‒gut‒brain axis is increasingly recognized as a critical pathway in the pathogenesis of depression and the response to antidepressant treatments. Esketamine(S-Ket), a noncompetitive glutamatergic N-methyl-D-aspartate receptor (NMDAR) antagonist, has shown a rapid and long-lasting antidepressant effects. However, the precise mechanisms underlying the antidepressant actions of S-Ket remain unclear.
Methods: In this study, we explore the role of gut microbiota and metabolites in the antidepressant effects of esketamine in lipopolysaccharide (LPS)-induced mouse model of depression. Behavioral tests, including the open-field test, forced swimming test, tail suspension test, and sucrose preference test, were conducted to evaluate the antidepressant efficacy of S-Ket. Additionally,16S rRNA sequencing and untargeted metabolomics were performed to characterize the gut microbiota and metabolome profiles in fecal and hippocampal tissues of LPS-induced mice treated with S-Ket. Bioinformatics analysis was employed to identify key changes. Spearman's rank correlation analyses were used to explore associations between depression-like behaviors (DLBs), differential gut microbes, and metabolites.
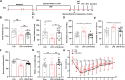
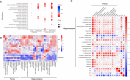
Results: S-Ket significantly alleviated DLBs in LPS-induced mice, partially restored the disrupted gut microbiota composition (β-diversity), and improved metabolic dysfunction. Pathway analysis revealed that four key amino acid metabolism pathways were significantly altered in both fecal and hippocampal samples, including ‘Glutathione metabolism’. ‘Alanine, aspartate, and glutamate metabolism’, ‘Arginine biosynthesis’, and ‘Arginine and proline metabolism’. Further analysis indicated that the genus Rikenella was significantly correlated with DLBs and host amino acids (e.g., glutamic acid and pyro glutamic acid).
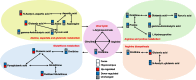
Conclusions: This study demonstrates that a single dose of S-Ket rapidly alleviates depression-like behaviors in LPS-induced mice, and its mechanisms are associated with regulating both the composition of gut microbiota and associated metabolites in fecal and hippocampal tissues, particularly the alteration of host amino acid metabolism. These results highlight the potential role of the gut–microbiome–amino acid metabolism axis in esketamine’s antidepressant effects and suggest that targeting this axis may offer therapeutic benefits for depression. Further research is needed to fully elucidate these mechanisms.
Graphical Abstract:
Supplementary Information: The online version contains supplementary material available at 10.1186/s12866-025-04181-3.
Keywords: Depression; Esketamine; Gut microbiota; Lipopolysaccharide; Metabolomics; Microbiota-Gut-Brain Axis.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Evans-Lacko S, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, Benjet C, Bruffaerts R, et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: results from the WHO World Mental Health (WMH) surveys. Psychol Med. 2018;48(9):1560–71. 10.1017/S0033291717003336. - DOI - PMC - PubMed
-
- Yuan M, Yang B, Rothschild G, Mann JJ, Sanford LD, Tang X, et al. Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct Target Ther. 2023;8(1):309. 10.1038/s41392-023-01519-z. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

