Characterization of odorant binding and chemosensory protein families in the kissing bug Triatoma infestans: comparative analysis among Heteroptera species
- PMID: 40890621
- PMCID: PMC12403313
- DOI: 10.1186/s12864-025-11967-2
Characterization of odorant binding and chemosensory protein families in the kissing bug Triatoma infestans: comparative analysis among Heteroptera species
Abstract
Background: Plasticity in sensory perception and tolerance to xenobiotics contributes to insects' adaptive capacity and evolutionary success, by enabling them to cope with potentially toxic molecules from the environment or internal milieu. Odorant-binding proteins (OBPs) and chemosensory proteins (CSPs) have traditionally been studied in the context of chemoreception. However, accumulating evidence over the past few years indicates that these protein families can also sequester insecticide molecules. In doing so, the insecticide cannot reach its target site and can be more easily eliminated through the feces, complexed with these proteins. Thus, xenobiotic sequestration by OBPs and CSPs may lead to insecticide tolerance or even resistance. In the Southern Cone, the kissing bug Triatoma infestans is the main vector of Trypanosoma cruzi, the protozoan parasite that causes Chagas disease. Vectorial transmission of T. cruzi has not been interrupted in certain regions of Argentina, where several populations of T. infestans highly resistant to insecticides have been reported. Understanding the molecular mechanisms underlying resistance is crucial for designing effective vector control strategies. In this context, studying protein families involved in insecticide sequestration is essential.
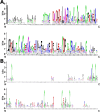
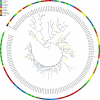
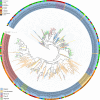
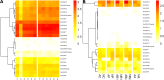
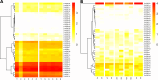
Results: We manually corrected predicted gene models and identified new sequences of chemosensory and odorant-binding proteins in five Hemiptera species with different feeding habits. Using this information, we mined the raw genome sequence of T. infestans to identify and characterize their orthologs based on sequence conservation and phylogenetic relationships. In total, 26 chemosensory and 49 odorant-binding proteins were identified in the T. infestans genome. Phylogenetic analysis, tissue-specific expression, and molecular docking with major insecticides were performed to assess possible roles.
Conclusions: This work represents the first comprehensive genomic analysis of chemosensory and odorant-binding protein families across Hemiptera species, as well as the first characterization of these gene families in T. infestans using genomic data. It contributes to a better understanding of the molecular basis of chemoreception and insecticide resistance in T. infestans.
Keywords: Chemoreception; Insecticide; Insects; Kissing bugs; Xenobiotic sequestration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All methods were carried out in accordance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

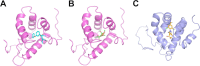

References
-
- Gadenne C, Barrozo RB, Anton S. Plasticity in Insect Olfaction: To Smell or Not to Smell? Annu. Rev. of Entomol., vol. 61. Annual Reviews Inc., pp. 317–333, 11-Mar-2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

