Efficacy and Safety of Tenecteplase in Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials
- PMID: 40891051
- PMCID: PMC12402402
- DOI: 10.1002/brb3.70791
Efficacy and Safety of Tenecteplase in Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials
Abstract
Introduction: Acute ischemic stroke (AIS) is the most common type of stroke, with increasing incidence and significant healthcare costs. Tenecteplase (TNK), a modified variant of tissue plasminogen activator (tPA), offers advantages such as a longer half-life and single-bolus administration. This meta-analysis evaluates the safety and efficacy of TNK compared to non-thrombolytic management in AIS to guide clinical decision-making.
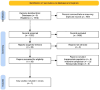
Methodology: A comprehensive literature search across major databases identified randomized controlled trials (RCTs) comparing tenecteplase with non-thrombolytic care in ischemic stroke. Data extraction and bias assessment were conducted independently, using RoB 2.0 and the GRADE framework. Meta-analysis was performed using RevMan 5.4.1, applying random-effects models and assessing heterogeneity with the I2 statistic.
Results: This meta-analysis included seven studies with 3266 patients and found no significant difference between tenecteplase and standard medical care in terms of the mRS score at 90 days (mean difference = -0.16, p = 0.58), functional independence (mRS 0-2 at 90 days) (odds ratio = 1.07, p = 0.51), and reperfusion (TICI 2b-3 at 24 h) (odds ratio = 1.33, p = 0.39). However, tenecteplase was associated with significantly higher mRS 0-1 at 90 days (odds ratio = 1.22, p = 0.01), better recanalization at 24 h (odds ratio = 3.28, p = 0.04), and improved NIHSS scores at 7 days (mean difference = -0.71, p = 0.003). On the downside, tenecteplase showed a significantly higher incidence of symptomatic intracranial hemorrhage (SICH) within 36 h (odds ratio = 2.24, p = 0.04) and any ICH (odds ratio = 1.40, p = 0.04), with no significant differences in mortality at 90 days (odds ratio = 1.18, p = 0.33) or stroke recurrence (odds ratio = 1.23, p = 0.55) and Barthel Index Score (odds ratio = 1.09, p = 0.69) and quality of life. Serious adverse events were slightly higher in the tenecteplase group but did not reach statistical significance (odds ratio = 1.18, p = 0.23).
Conclusion: Tenecteplase improves early neurological recovery and recanalization and provides excellent functional outcomes in acute ischemic stroke. However, it is associated with a higher risk of symptomatic and overall intracranial hemorrhage. Mortality, stroke recurrence, and overall functional independence remain unaffected.
Keywords: acute ischemic stroke (AIS); hemorrhage; recanalization; tenecteplase (TNK); thrombolysis.
© 2025 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Baig, M. U. , and Bodle J.. 2023. Thrombolytic Therapy. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK557411/. - PubMed
-
- Campbell, B. C. V. , Mitchell P. J., Churilov L., et al. 2020. “Effect of Intravenous Tenecteplase Dose on Cerebral Reperfusion Before Thrombectomy in Patients With Large Vessel Occlusion Ischemic Stroke: The EXTEND‐IA TNK Part 2 Randomized Clinical Trial.” JAMA 323, no. 13: 1257–1265. 10.1001/jama.2020.1511. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

