Azelastine Nasal Spray for Prevention of SARS-CoV-2 Infections: A Phase 2 Randomized Clinical Trial
- PMID: 40892398
- PMCID: PMC12406145
- DOI: 10.1001/jamainternmed.2025.4283
Azelastine Nasal Spray for Prevention of SARS-CoV-2 Infections: A Phase 2 Randomized Clinical Trial
Erratum in
-
Error in Figure 1 and Discussion Section.JAMA Intern Med. 2025 Nov 1;185(11):1401. doi: 10.1001/jamainternmed.2025.5850. JAMA Intern Med. 2025. PMID: 41182786 Free PMC article. No abstract available.
Abstract
Importance: Limited pharmaceutical options exist for preexposure prophylaxis of COVID-19 beyond vaccination. Azelastine, an antihistamine nasal spray used for decades to treat allergic rhinitis, has in vitro antiviral activity against respiratory viruses, including SARS-CoV-2.
Objective: To determine the efficacy and safety of azelastine nasal spray for prevention of SARS-CoV-2 infections in healthy adults.
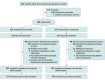
Design, setting, and participants: A phase 2, double-blind, placebo-controlled, single-center trial was conducted from March 2023 to July 2024. Healthy adults from the general population were enrolled at the Saarland University Hospital in Germany.
Interventions: Participants were randomly assigned 1:1 to receive azelastine, 0.1%, nasal spray or placebo 3 times daily for 56 days. SARS-CoV-2 rapid antigen testing (RAT) was conducted twice weekly, with positive results confirmed by polymerase chain reaction (PCR). Symptomatic participants with negative RAT results underwent multiplex PCR testing for respiratory viruses.
Main outcome: The primary end point was the number of PCR-confirmed SARS-CoV-2 infections during the study.
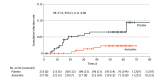
Results: A total of 450 participants were randomized, with 227 assigned to azelastine and 223 to placebo; 299 (66.4%) were female, 151 (33.6%) male, with a mean (SD) age of 33.0 (13.3) years. Most were White (417 [92.7%]), with 4 (0.9%) African, 22 (4.9%) Asian, and 7 (1.6%) of other ethnicity. In the intention-to-treat (ITT) population, the incidence of PCR-confirmed SARS-CoV-2 infection was significantly lower in the azelastine group (n = 5 [2.2%]) compared with the placebo group (n = 15 [6.7%]) (OR, 0.31; 95% CI, 0.11-0.87). As secondary end points, azelastine demonstrated an increase in mean (SD) time to SARS-CoV-2 infection among infected participants (31.2 [9.3] vs 19.5 [14.8] days), a reduction of the overall number of PCR-confirmed symptomatic infections (21 of 227 participants vs 49 of 223 participants), and a lower incidence of PCR-confirmed rhinovirus infections (1.8% vs 6.3%). Adverse events were comparable between the groups.
Conclusions and relevance: In this single-center trial, azelastine nasal spray was associated with reduced risk of SARS-CoV-2 respiratory infections. These findings support the potential of azelastine as a safe prophylactic approach warranting confirmation in larger, multicentric trials.
Trial registration: EudraCT number: 2022-003756-13.
Conflict of interest statement
Figures
Comment on
-
A Novel Approach for Preventing Respiratory Virus Infections.JAMA Intern Med. 2025 Nov 1;185(11):1317-1318. doi: 10.1001/jamainternmed.2025.4297. JAMA Intern Med. 2025. PMID: 40892421 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

