Maladaptive trained immunity in viral infections
- PMID: 40892509
- PMCID: PMC12404746
- DOI: 10.1172/JCI192469
Maladaptive trained immunity in viral infections
Abstract
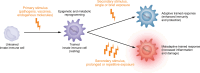
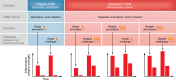
Trained immunity (TRIM) is a form of long-lasting functional reprogramming of innate immune cells and their progenitors that enhances responsiveness to subsequent stimuli. Although first characterized in myeloid cells, TRIM was recently extended to nonmyeloid cell types, including endothelial and glial cells, which also exhibit stimulus-driven, memory-like behavior. While initially recognized as a protective mechanism, particularly in the context of vaccines and acute infections, TRIM can also become maladaptive, promoting chronic inflammation, immune dysfunction, and disease. This Review focuses on virus-induced TRIM while also addressing microbial, metabolic, and endogenous inducers. We examine key ligands and receptors that initiate TRIM and dissect the associated signaling and epigenetic pathways. Importantly, we argue that maladaptive TRIM arises not from a specific ligand, receptor, or molecular event, but from contextual factors such as stimulus persistence, dose, tissue microenvironment, and preexisting inflammation. The nature of the secondary challenge also shapes whether a trained response is adaptive or maladaptive. We further discuss TRIM induction in the bone marrow, involvement of both myeloid and nonmyeloid cells, and the role of lipid rafts in sustaining TRIM. We review maladaptive TRIM's potential contribution to systemic diseases, such as atherosclerosis, diabetes, sepsis, cancer, and autoimmunity, along with its influence on viral vaccine responses. Finally, we outline potential strategies to redirect maladaptive TRIM and propose key outstanding questions for future research.
Conflict of interest statement
Figures
References
-
- Lu Y, et al. Increased acetylation of H3K14 in the genomic regions that encode trained immunity enzymes in lysophosphatidylcholine-activated human aortic endothelial cells — novel qualification markers for chronic disease risk factors and conditional DAMPs. Redox Biol. 2019;24:101221. doi: 10.1016/j.redox.2019.101221. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

