Potential Role of SGLT-2 Inhibitors in Improving Allograft Function and Reducing Rejection in Kidney Transplantation
- PMID: 40892675
- PMCID: PMC12404283
- DOI: 10.1111/ctr.70233
Potential Role of SGLT-2 Inhibitors in Improving Allograft Function and Reducing Rejection in Kidney Transplantation
Abstract
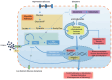
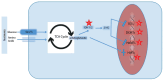
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) have demonstrated renoprotective and cardioprotective benefits beyond their antiglycemic effects. Their potential utility in kidney transplant recipients (KTRs) for preserving graft function and reducing rejection risk is currently under active investigation. Preliminary studies indicate that SGLT-2i therapy stabilizes estimated glomerular filtration rate (eGFR), decreases glomerular hyperfiltration, and improves metabolic outcomes in KTRs. Emerging clinical evidence also suggests that SGLT-2i may be associated with reduced rates of acute rejection, although direct immunosuppressive actions remain unclear. Experimental findings further suggest that SGLT-2i modulates gene regulation pathways involved in inflammation, oxidative stress, and fibrosis, contributing to improved allograft outcomes. Current safety data in KTRs are reassuring, without significant increases in urinary tract infections or adverse graft events. Nevertheless, long-term prospective studies specific to transplant populations are lacking. This review summarizes available evidence regarding the mechanisms of action, clinical efficacy, and safety profile of SGLT-2i in kidney transplantation, emphasizing their metabolic, hemodynamic, inflammatory, and immunomodulatory effects.
Keywords: immunosuppressant; mechanistic target of rapamycin (mTOR); rejection; signaling/signaling pathways.
© 2025 The Author(s). Clinical Transplantation published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Neuberger J. M., Bechstein W. O., Kuypers D. R., et al., “Practical Recommendations for Long‐Term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group,” Transplantation 101, no. 4S Suppl 2 (2017): S1–S56. - PubMed
-
- Van Loon E., Bernards J., Van Craenenbroeck A. H., and Naesens M., “The Causes of Kidney Allograft Failure: More Than Alloimmunity. A Viewpoint Article,” Transplantation 104, no. 2 (2020): e46–e56. - PubMed
-
- Gaston R. S., Fieberg A., Helgeson E. S., et al., “Late Graft Loss After Kidney Transplantation: Is “Death With Function” Really Death With a Functioning Allograft?” Transplantation 104, no. 7 (2020): 1483–1490. - PubMed
-
- Rodrigues C. A., Franco M. F., Cristelli M. P., Pestana J. O., and Tedesco‐Silva H. Jr., “Clinicopathological Characteristics and Effect of Late Acute Rejection on Renal Transplant Outcomes,” Transplantation 98, no. 8 (2014): 885–892. - PubMed
-
- Redondo‐Pachon D., Calatayud E., Buxeda A., et al., “Evolution of Kidney Allograft Loss Causes Over 40 Years (1979‐2019),” Nefrologia (Engl Ed) 43, no. 3 (2023): 316–327. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

