AhR signaling in skin-resident CD207+ cells is involved in UV-B-induced amelioration of neuroinflammation
- PMID: 40892929
- PMCID: PMC12435267
- DOI: 10.1073/pnas.2424009122
AhR signaling in skin-resident CD207+ cells is involved in UV-B-induced amelioration of neuroinflammation
Abstract
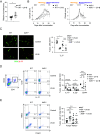
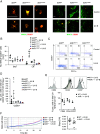
Environmental stimuli, including the exposure to ultraviolet (UV)-B light, are known to play a role in the modulation of immune-mediated mechanisms in multiple sclerosis (MS). In experimental autoimmune encephalomyelitis (EAE), we have shown that UV-B irradiation ameliorates disease outcome by regulatory T cells (Treg) expansion. Moreover, the UV-B-mediated induction of Treg numbers was also observed in MS. The aryl hydrocarbon receptor (AhR) can be activated by environmental factors including UV-B-induced photoproducts of tryptophan. Thus, we investigated the role of AhR during the transmission of UV-B irradiation. Therefore, wild-type (WT) and AhR-deficient mice (AhR-/-) were irradiated with UV-B light and immunized with myelin oligodendrocyte glycoprotein (MOG)-peptide. In WT mice it was shown that UV-B irradiation reduces EAE symptoms by Treg expansion. This effect was abrogated in animals with AhR deficiency. To better understand the underlying mechanisms of AhR regulation, we used mice with a deletion of AhR specifically in different subsets of antigen-presenting cells (APC) that have been shown to mediate the expansion of Treg. Interestingly, we could show that the AhR activation in murine cutaneous APC was sufficient to switch APC from a stimulatory into a regulatory phenotype, and moreover, responsible for APC cell maturation and migration into regional lymph nodes. Thus, our data indicate that AhR activation in APC might be required for UV-B-mediated immunosuppression during MOG-induced EAE. Hence, activation of AHR in tissue-resident APC, potentially by low-dose UV-B irradiation, might be beneficial as an adjuvant treatment in inflammatory or degenerative diseases of the central nervous system.
Keywords: UV-B; aryl hydrocarbon receptor; cutaneous dendritic cells; experimental autoimmune encephalomyelitis; multiple sclerosis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Breuer J., et al. , Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann. Neurol. 75, 739–758 (2014). - PubMed
-
- Lincoln J. A., Cook S. D., An overview of gene-epigenetic-environmental contributions to MS causation. J. Neurol. Sci. 286, 54–57 (2009). - PubMed
-
- Oberg M., Bergander L., Håkansson H., Rannug U., Rannug A., Identification of the tryptophan photoproduct 6-formylindolo[3, 2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol. Sci. 85, 935–943 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

