Impact of renal function on the efficacy of low-voltage area ablation after pulmonary vein isolation: a sub-analysis of the SUPPRESS-AF trial
- PMID: 40892973
- PMCID: PMC12448949
- DOI: 10.1093/europace/euaf205
Impact of renal function on the efficacy of low-voltage area ablation after pulmonary vein isolation: a sub-analysis of the SUPPRESS-AF trial
Abstract
Aims: The SUPPRESS-AF trial showed that pulmonary vein isolation (PVI) plus low-voltage area (LVA) ablation may reduce atrial fibrillation (AF) recurrence in some subgroups. Renal dysfunction is a cause of LVAs due to atrial cardiomyopathy and is also a risk factor for AF recurrence after catheter ablation. The aim of this study was to investigate the efficacy of LVA ablation after PVI stratified by renal function.
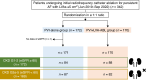
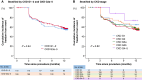
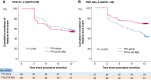
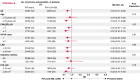
Methods and results: This study was a sub-analysis of the SUPPRESS-AF trial, a multicentre, prospective, randomized, open-label trial. A total of 341 consecutive patients who underwent initial radiofrequency catheter ablation for persistent AF and whose LVAs were ≥5 cm2 were analysed. Patients were randomized to PVI alone (PVI-alone group) or LVA ablation after PVI [PVI + LVA-ablation (ABL) group]. Primary outcome was defined as the recurrence of atrial tachyarrhythmias during the 12 months following ablation. Estimated glomerular filtration rate (eGFR) was assessed before ablation, and patients were stratified by chronic kidney disease (CKD) stage. The mean eGFR was 60 ± 16 mL/min/1.73 m2, and 146 (43%) patients developed the primary outcome. In patients with CKD G1-2 (eGFR ≥ 60 mL/min/1.73 m2), freedom from the primary outcome was similar between the PVI + LVA-ABL and PVI-alone groups (53.1% vs. 55.3%, P = 0.59). In contrast, in patients with CKD G3a-5 (eGFR < 60 mL/min/1.73 m2), freedom from the primary outcome was significantly higher in the PVI + LVA-ABL group than in the PVI-alone group (69.1% vs. 43.3%; P = 0.004).
Conclusion: In patients with renal dysfunction, LVA ablation after PVI reduced AF recurrence after radiofrequency catheter ablation for persistent AF.
Keywords: Atrial fibrillation; Catheter ablation; Chronic kidney disease; Left atrial remodelling; Renal function.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: Y.M. has received a grant from the Japanese Heart Rhythm Society, Abbott, and Nihon Kohden outside the submitted work; M.M. has received personal fees from Daiichi Sankyo, Medtronic, Johnson & Johnson, and Boston Scientific outside the submitted work; T.I. has received honoraria from Nipro and Kaneka Medics outside the submitted work; N.T. has received personal fees from Daiichi Sankyo, Bayer, Boehringer Ingelheim Japan, AstraZeneca, Johnson & Johnson K.K., and Medtronic outside the submitted work; H.M. has received personal fees from Medtronic, Abbott, Nihon Kohden, Biotronik, Japan Lifeline, Boston Scientific, Philips, Cook Medical, Bayer, Daiichi Sankyo, Johnson & Johnson, and Microport outside the submitted work; Y.E. has received personal fees from Abbott, Johnson & Johnson, Japan Lifeline, and Medtronic outside the submitted work; T.O. has received personal fees from Beyer and Medtronic outside the submitted work; T.K. has received personal fees from Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Nihon Kohden, Abbott, Medtronic, and Otsuka Pharmaceutical, outside the submitted work; M.Ka. has received personal fees from Medtronic, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Bristol-Myers Squibb, and Abbott outside the submitted work; N.M. has received personal fees from Biosense Webster, Abbott, Medtronic, Bristol-Myers Squibb, Boehringer Ingelheim, Bayer, Daiichi Sankyo, and Otsuka Pharmaceutical outside the submitted work; S.H. has received grants from Medtronic, Johnson & Johnson, and Abbott during the conduct of the study, personal fees from Bayer, Daiichi Sankyo, Medtronic, Boehringer Ingelheim, Johnson & Johnson, Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion; and non-financial support from Actelion outside the submitted work; T.D. has received grants from Medtronic, Johnson & Johnson, and Abbott during the conduct of the study; K.I. has received personal fee from Daiichi Sankyo, Johnson & Johnson, Boston Scientific, and Medtronic outside the submitted work; Y.So. has received grants from Abbott Medical Japan, Abiomed, Biosense Webster, Boston Scientific Japan, Bristol-Myers Squibb, FUJIFILM Toyama Chemical, Roche Diagnostics, Terumo, TOA EIYO, and Nipro and personal fees from Abbott Medical Japan, Amgen Astellas BioPharma, Bristol-Myers Squibb, and Shockwave outside the submitted work; Y.Sa. has received grants from Medtronic, Johnson & Johnson, and Abbott during the conduct of the study and personal fees from Abbott, Sanofi, Johnson & Johnson, Daiichi Sankyo, Terumo, Medtronic, Bayer, Biotronik, Bristol-Myers Squibb, Boehringer Ingelheim, and Boston Scientific outside the submitted work. T.Ma., T.T., H.U., H.O., S.K., M.Oc., S.O., K.N., Y.H., S.N., M.Ku., W.A., A.S., T.W., T.Mi., M.Ok., K.T., and H.K. have no conflicts of interest to declare.
Figures


References
-
- Tzeis S, Gerstenfeld EP, Kalman J, Saad EB, Sepehri Shamloo A, Andrade JG et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024;26:euae043.
-
- Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai K et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ J 2021;85:1104–244. - PubMed
-
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. - PubMed
-
- Masuda M, Matsuda Y, Uematsu H, Sugino A, Ooka H, Kudo S et al. Prognostic impact of atrial cardiomyopathy: long-term follow-up of patients with and without low-voltage areas following atrial fibrillation ablation. Heart Rhythm 2024;21:378–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

