AI-Driven Tacrolimus Dosing in Transplant Care: Cohort Study
- PMID: 40893105
- PMCID: PMC12404564
- DOI: 10.2196/67302
AI-Driven Tacrolimus Dosing in Transplant Care: Cohort Study
Abstract
Background: Tacrolimus forms the backbone of immunosuppressive therapy in solid organ transplantation, requiring precise dosing due to its narrow therapeutic range. Maintaining therapeutic tacrolimus levels in the postoperative period is challenging due to diverse patient characteristics, donor organ factors, drug interactions, and evolving perioperative physiology.
Objective: The aim of this study is to design a machine learning model to predict the next-day tacrolimus trough concentrations (C0) and guide dosing to prevent persistent under- or overdosing.
Methods: We used retrospective data from 1597 adult recipients of kidney and liver transplants at UC San Diego Health to develop a long short-term memory (LSTM) model to predict next-day tacrolimus C0 in an inpatient setting. Predictors included transplant type, demographics, comorbidities, vital signs, laboratory parameters, ordered diet, and medications. Permutation feature importance was evaluated for the model. We further implemented a classification task to evaluate the model's ability to identify underdosing, therapeutic dosing, and overdosing. Finally, we generated next-day dose recommendations that would achieve tacrolimus C0 within the target ranges.
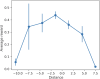
Results: The LSTM model provided a mean absolute error of 1.880 ng/mL when predicting next-day tacrolimus C0. Top predictive features included the recent tacrolimus C0, tacrolimus doses, transplant organ type, diet, and interactive drugs. When predicting underdosing, therapeutic dosing, and overdosing using a 3-class classification task, the model achieved a microaverage F1-score of 0.653. For dose recommendations, the best clinical outcomes were achieved when the actual total daily dose closely aligned with the model's recommended dose (within 3 mg).
Conclusions: Ours is one of the largest studies to apply artificial intelligence to tacrolimus dosing, and our LSTM model effectively predicts tacrolimus C0 and could potentially guide accurate dose recommendations. Further prospective studies are needed to evaluate the model's performance in real-world dose adjustments.
Keywords: clinical decision-making; machine learning; organ transplantation; personalized medicine; tacrolimus.
© Mingjia Huo, Sean Perez, Linda Awdishu, Janice S Kerr, Pengtao Xie, Adnan Khan, Kristin Mekeel, Shamim Nemati. Originally published in JMIR AI (https://ai.jmir.org).
Conflict of interest statement
Figures

References
-
- Alcamo AM, Clark RSB, Au AK, et al. Factors associated with neurobehavioral complications in pediatric abdominal organ transplant recipients identified using computable composite definitions. Pediatr Crit Care Med. 2020 Sep;21(9):804–810. doi: 10.1097/PCC.0000000000002355. doi. Medline. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

