Selective Green Colorimetric Liquid- and Solid-Based Determination of Furfural in Power Transformer Insulating Fluid
- PMID: 40893314
- PMCID: PMC12392036
- DOI: 10.1021/acsomega.5c00572
Selective Green Colorimetric Liquid- and Solid-Based Determination of Furfural in Power Transformer Insulating Fluid
Abstract
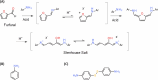
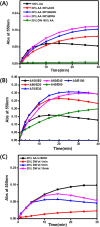
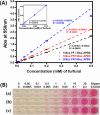
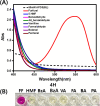
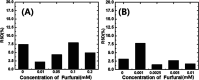
Furfural (FF), an intermediate aldehyde compound, can serve as an indicator of the extent of the Maillard reaction on heat-induced food processing and storage, as well as for the aging assessment of the solid insulation of oil-immersed transformers because it is formed by the degradation of cellulosic insulation. By considering the concepts of green analytical chemistry, a new furfural-bis-(4-aminophenyl) disulfide (APDS) colorimetric chemosensory assay, based on the Stenhouse reaction between furfural and APDS, was developed for the quantification of furfural by utilizing UV spectroscopy and colorimetric analyses. A strong correlation between UV-vis absorbance and furfural concentrations was observed, which confirmed the high sensitivity (0.00024 mM furfural) of the reaction system. The color change at 0.005 mM of furfural was noted by the naked eye. This was a unique and highly selective phenomenon because only furfural shows UV absorbance and color change within 450-600 nm of UV radiation, unlike other aromatic and aliphatic aldehydes. The highly sensitive method was applied for the qualification of 0-0.01 mM (0-1 ppm) of furfural in the power transformer insulating fluid. An APDS strip formulated with polyethylene glycol was used as the chromatography paper. This study demonstrates the successful surface Stenhouse coupling between furfural and APDS, as confirmed by X-ray photoelectron spectroscopy analysis. Therefore, this liquid- and solid-based assay is a novel, green analytical method as it uses safer solid APDS instead of toxic liquid aniline that is generally used in conventional methods. In addition, the method is simpler, which makes the on-site analysis possible.
© 2025 The Authors. Published by American Chemical Society.
Figures





References
-
- Anastas P. T.. Green chemistry and the role of analytical methodology development. Crit. Rev. Anal. Chem. 1999;29:167–175. doi: 10.1080/10408349891199356. - DOI
-
- Gałuszka A., Migaszewski Z., Namieśnik J.. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC, Trends Anal. Chem. 2013;50:78–84. doi: 10.1016/j.trac.2013.04.010. - DOI
-
- Rico-Yuste A., González-Vallejo V., Benito-Peña E., De las Casas Engel T., Orellana G., Moreno-Bondi M. C.. Furfural determination with disposable polymer films and smartphone-based colorimetry for beer freshness assessment. Anal. Chem. 2016;88:3959–3966. doi: 10.1021/acs.analchem.6b00167. - DOI - PubMed
LinkOut - more resources
Full Text Sources
