The role of non-linear viscoelastic hydrogel mechanics in cell culture and transduction
- PMID: 40893357
- PMCID: PMC12391783
- DOI: 10.1016/j.mtbio.2025.102188
The role of non-linear viscoelastic hydrogel mechanics in cell culture and transduction
Abstract
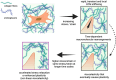
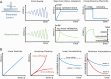
The mechanical complexity of the extracellular matrix (ECM) is central to how cells sense and respond to their environment, yet hydrogel design has often focused narrowly on stiffness. Emerging evidence highlights the importance of viscoelastic stress relaxation and plasticity in cell mechanotransduction. However, a key aspect remains underexplored: non-linear viscoelasticity, where stress relaxation and plasticity depend on the magnitude of applied stress or strain. In this perspective, we examine how such non-linear mechanical behaviors manifest in widely used hydrogels and discuss their biological relevance. We present experimental approaches, including oscillatory shear rheology, to detect non-linear viscoelastic effects, and introduce mathematical modeling approaches to interpret these behaviors. We find evidence in literature that several hydrogels commonly used in cell culture exhibit non-linear viscoelasticity occurring at stress and strain levels relevant to cell-generated forces. Specifically, both softening and stiffening hydrogels were found to exhibit accelerated stress relaxation or increased plasticity due to nonlinear viscoelasticity. By viewing non-linearity as a tunable design parameter, future hydrogel systems may better recapitulate the dynamic mechanical feedback loops cells experience in native tissues. This perspective encourages a paradigm shift in biomaterial design, integrating non-linear viscoelasticity into the next generation of ECM-mimetic hydrogels for cell culture and regenerative applications.
Keywords: Cell biology; Hydrogels; Mathematical modelling; Mechanotransduction; Non-linear viscoelasticity; Rheology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

