Recent advances in copper sulfide nanoparticles for cancer diagnosis and therapy
- PMID: 40893366
- PMCID: PMC12391698
- DOI: 10.1016/j.mtbio.2025.102197
Recent advances in copper sulfide nanoparticles for cancer diagnosis and therapy
Abstract
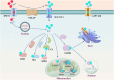
Cancer, a highly heterogeneous and complex disease characterized by multiple genetic and metabolic abnormalities, remains one of the leading causes of death worldwide. Although conventional treatments such as surgery, chemotherapy, and radiotherapy, can mitigate the disease to some extent, their efficacy remains constrained by various factors. In recent years, nanotechnology has emerged as a promising approach for cancer treatment, with copper-based nanomaterials garnering significant attention due to their unique physicochemical properties and favorable biocompatibility. Copper sulfide (CuS) nanomaterials, in particular, have shown great potential as a versatile platform for both diagnosis and therapy, primarily due to their superior photothermal properties. Moreover, copper plays a crucial role in tumorigenesis, progression, and multiple cell death pathways, further highlighting its potential in cancer therapy. This review discusses the metabolic regulation of copper and its diverse roles in tumor biology, examines the applications and recent advances of CuS nanomaterials in cancer therapy, and explores their future potential in cancer diagnosis and treatment.
Keywords: Copper sulfide nanomaterials; Cuproptosis; Multimodal combination therapy; Nanomedicine; Photothermal therapy.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures














References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2024;74:229–263. - PubMed
-
- Kaur R., Bhardwaj A., Gupta S. Cancer treatment therapies: traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023;50:9663–9676. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

