Phosphorus-32 microspheres: A dual-modality transarterial radioembolization approach for hepatocellular carcinoma therapy and Anti-PD1 immunotherapy potentiation
- PMID: 40893370
- PMCID: PMC12395517
- DOI: 10.1016/j.mtbio.2025.102210
Phosphorus-32 microspheres: A dual-modality transarterial radioembolization approach for hepatocellular carcinoma therapy and Anti-PD1 immunotherapy potentiation
Abstract
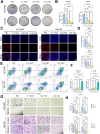
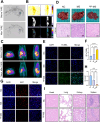
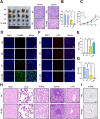
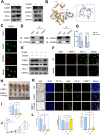
Transarterial radioembolization (TARE) is a key therapy for hepatocellular carcinoma (HCC) management and downstaging. While 90Y microspheres (glass/resin) are widely used, their clinical application is limited by complexity, short half-life, and high costs. Thus, novel radionuclide microspheres are crucial. This study developed phosphorus-32-loaded microspheres (32P-MS). In vitro, 32P-MS dose-dependently suppressed HCC cell proliferation, migration, and invasion while inducing apoptosis. In vivo, 32P-MS TARE achieved tumor vascular embolization, reducing tumor vol/wt (confirmed by Positron Emission Tomography-Computed Tomography (PET-CT), Hematoxylin and Eosin (HE) staining, TUNEL/Ki67 assays without systemic toxicity. RNA sequencing and mass cytometry analyses revealed 32P-MS upregulated FABP1+PD-L1+ myeloid-derived suppressor cell (MDSC), linked to immunosuppression. Mechanistic investigations, including molecular docking, co-localization, and co-immunoprecipitation (Co-IP) assays, demonstrated that 32P-MS activated the FABP1/PPARG/PD-L1 axis in MDSC. Genetic ablation of FABP1 or pharmacological inhibition with Orlistat reversed PD-L1 expression and augmented anti-tumor efficacy. Combining 32P-MS with anti-PD1 therapy synergistically suppressed tumor growth, reduced MDSC infiltration, and reinvigorated CD8+ T cell activity, significantly improving treatment sensitivity. 32P-MS is a promising HCC therapeutic with dual anti-tumor and immune-modulatory functions, providing a compelling rationale for integrating radioembolization with immune checkpoint blockade to counteract immunosuppressive resistance in HCC.
Keywords: Hepatocellular carcinoma; Myeloid-derived suppressor cell; PD1; Phosphorus-32-loaded microspheres; Transarterial radioembolization.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J. Clin. 2019;69(1):7–34. 2019. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

