Imaging biomarkers in antibody-mediated autoimmune encephalitis
- PMID: 40893523
- PMCID: PMC12397707
- DOI: 10.21037/qims-2025-131
Imaging biomarkers in antibody-mediated autoimmune encephalitis
Abstract
Background: Imaging, particularly multimodal magnetic resonance imaging (MRI), serves as an essential auxiliary examination for diagnosing autoimmune encephalitis (AE). The diversity of autoantibodies complicates the imaging presentation of AE, exhibiting both common and individual features across different subtypes of AE. Currently, there is a lack of comprehensive studies on the imaging features of different subtypes of AE. The study aimed to explore imaging biomarkers for AE mediated by various subtypes of antibodies and clarify their significance in disease severity, treatment response, and prognosis.
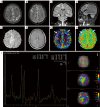
Methods: The clinical and imaging data of 45 patients with AE at The First Affiliated Hospital of Dalian Medical University, collected from January 2013 to August 2022, were analyzed. Patients underwent multi-modal brain MRI. Lesion probability maps were generated, and regions of interest (ROIs) were selected based on lesion location and clinical-electroencephalographic features, for measurement of three-dimensional T1-weighted imaging (3D-T1WI), T2-weighted imaging (T2WI), T2 fluid-attenuated inversion recovery (T2 FLAIR), and apparent diffusion coefficient (ADC) sequences. These values were used for correlating with disease severity, antibody titers, response to treatment, and prognosis.
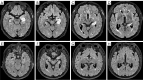
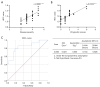
Results: The study included 45 AE patients: 18 with anti-leucine-rich glioma inactivated protein 1 (anti-LGI1), 11 with anti-N-methyl-D-aspartate receptor (anti-NMDAR), 5 with anti-gamma-aminobutyric acid receptor B (anti-GABABR), 4 with anti-myelin oligodendrocyte glycoprotein (MOG), 4 with anti-glutamate decarboxylase 65 (anti-GAD65), and 3 with anti-contactin-associated protein-like 2 (anti-Caspr2) encephalitis. MRI abnormalities were present in 62.2% of patients, lower than that of electroencephalography (EEG) (95.6%, P<0.05). Imaging typically showed common features across different AE subtypes, predominantly involving the limbic system or regions outside of it, manifesting as T1 hypointensity, T2 FLAIR hyperintensity or mild hyperintensity, and normal or mild hyperintensity on diffusion-weighted imaging (DWI). Different AE subtypes displayed specific imaging features: anti-LGI1 encephalitis often involved 2 locations: unilateral or bilateral hippocampus or basal ganglia; anti-NMDAR encephalitis showed a low rate of imaging abnormalities, with diffuse and unfixed cortical or subcortical T2 FLAIR hyperintensity. Anti-GABABR encephalitis primarily affected the temporal lobe or hippocampus. MOG antibody cortical encephalitis exhibited cortical swelling with T2 FLAIR hyperintensity in unilateral or bilateral hemispheres, particularly in the frontal lobe. Anti-GAD65 encephalitis involved the temporal lobe/hippocampus or pontocerebellar regions. The ADC value within the ROI positively correlated with both disease severity (r=0.6891, P<0.0001) and prognosis score (r=0.8102, P<0.0001). Further analysis using receiver operating characteristic (ROC) curve and binary logistic regression indicated that the ADC value was a risk factor for poor prognosis.
Conclusions: Imaging abnormalities are less frequent than those detected by EEG but exhibit distinct features by subtype. Functional imaging enhances diagnostic accuracy. ADC values can serve as a crucial prognostic indicator.
Keywords: Antibody-mediated autoimmune encephalitis (antibody-mediated AE); apparent diffusion coefficient value (ADC value); brain magnetic resonance imaging (brain MRI).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-2025-131/coif). Y.W. reports grants from the Liaoning Province Science and Technology Plan Project (grant No. 2024-MS-159). The other authors have no conflicts of interest to declare.
Figures





References
-
- Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25-36. 10.1002/ana.21050 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
