Development of a prediction model for HER2 low breast cancer using quantitative intra- and peri-tumoral heterogeneity and MRI features on high-spatial resolution ultrafast DCE-MRI
- PMID: 40893528
- PMCID: PMC12397656
- DOI: 10.21037/qims-24-976
Development of a prediction model for HER2 low breast cancer using quantitative intra- and peri-tumoral heterogeneity and MRI features on high-spatial resolution ultrafast DCE-MRI
Abstract
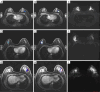
Background: Accurate preoperative human epidermal growth factor receptor 2 (HER2) status assessment is crucial for guiding treatment selection, particularly with the emergence of anti-HER2 antibody-drug conjugates (ADCs) for HER2-low breast cancer. However, current immunohistochemistry (IHC)-based classification is limited by spatial heterogeneity and sampling bias. Quantitative analysis of intra- and peri-tumoral heterogeneity (ITH) on imaging may offer a non-invasive, objective, and reproducible approach to distinguish HER2-low breast cancer from other subtypes. This study aimed to investigate quantitative ITH from high-spatial resolution ultrafast dynamic contrast-enhanced magnetic resonance imaging (UF DCE-MRI) based kinetic curves in distinguishing HER2 low from HER2 zero or positive breast cancer.
Methods: Consecutive breast cancer patients who underwent preoperative high-spatial-resolution UF DCE-MRI were retrospectively enrolled. They were stratified into HER2 zero, HER2 low, or HER2 positive groups based on IHC and in situ hybridization results. Traditional MRI findings and clinicopathological characteristics were evaluated, and personalized ITH scores were constructed using semi-quantitative parameters derived from kinetic curves. Models incorporating ITH, MRI, and clinicopathological distinctions were developed for dichotomized HER2 statuses prediction using multivariable logistic regression. The added value of ITH in the Final Combined Model was evaluated.
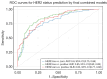
Results: This study enrolled 368 patients, with 45.9% (169/368) having HER2-low breast cancer. The ITH score was higher in HER2 low than that in HER2 zero (P<0.001), but lower than that in HER2 positive (P<0.001). The ITH score was higher in HER2 positive compared to HER2 zero (P<0.001). The Final Combined Model integrating ITH, MRI, and clinicopathological variables achieved good predictive performance, achieving area under the curve (AUC) values of 0.80 [95% confidence interval (CI): 0.75-0.86] for HER2 low vs. zero, 0.85 (95% CI: 0.80-0.89) for HER2 low vs. positive, and 0.83 (95% CI: 0.77-0.88) for HER2 zero vs. positive. The corresponding sensitivity/specificity values were 77%/72%, 77%/81%, and 94%/58%, respectively. The ITH score significantly enhanced HER2 status prediction, supported by AUC improvement (DeLong test, P<0.05), along with statistical significance in net reclassification improvement (NRI) (P<0.001) and integrated discrimination improvement (IDI) (P<0.001) across all tasks.
Conclusions: Integrating ITH from high-spatial resolution UF DCE-MRI-based kinetic curves improved the non-invasive differentiation of HER2-low breast cancer. This approach may guide targeted biopsy strategies and aid in selecting candidates for anti-HER2 ADC therapy, optimizing HER2-targeted precision medicine.
Keywords: Breast neoplasms; human epidermal growth factor receptor 2 (HER2); magnetic resonance imaging (MRI).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-976/coif). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
MRI-based habitat analysis for Intratumoral heterogeneity quantification combined with deep learning for HER2 status prediction in breast cancer.Magn Reson Imaging. 2025 Oct;122:110429. doi: 10.1016/j.mri.2025.110429. Epub 2025 May 23. Magn Reson Imaging. 2025. PMID: 40414575
-
Establishment of an interpretable MRI radiomics-based machine learning model capable of predicting axillary lymph node metastasis in invasive breast cancer.Sci Rep. 2025 Jul 18;15(1):26030. doi: 10.1038/s41598-025-10818-0. Sci Rep. 2025. PMID: 40676103 Free PMC article.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Dynamic contrast-enhanced MRI-based radiomics model of intra-tumoral kinetic heterogeneity for predicting breast cancer molecular subtypes.Front Mol Biosci. 2025 Jul 18;12:1635296. doi: 10.3389/fmolb.2025.1635296. eCollection 2025. Front Mol Biosci. 2025. PMID: 40756942 Free PMC article.
-
MRI-Based Radiomics Methods for Predicting Ki-67 Expression in Breast Cancer: A Systematic Review and Meta-analysis.Acad Radiol. 2024 Mar;31(3):763-787. doi: 10.1016/j.acra.2023.10.010. Epub 2023 Nov 2. Acad Radiol. 2024. PMID: 37925343
References
-
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ; Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. 10.1093/annonc/mdr304 - DOI - PMC - PubMed
-
- Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. 10.1200/JCO.2018.77.8738 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
