Improved prebiotic-based "shield" equipped probiotics for enhanced colon cancer therapy by polarizing M1 macrophages and regulating intestinal microbiota
- PMID: 40893693
- PMCID: PMC12399202
- DOI: 10.1016/j.apsb.2025.05.040
Improved prebiotic-based "shield" equipped probiotics for enhanced colon cancer therapy by polarizing M1 macrophages and regulating intestinal microbiota
Abstract
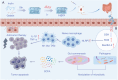
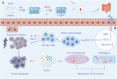
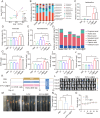
Probiotics play a crucial role in colon cancer treatment by metabolizing prebiotics to generate short-chain fatty acids (SCFAs). Colon cancer patients are frequently propositioned to supplement with probiotics to enhance the conversion and utilization of prebiotics. Nevertheless, the delivery and colonization of probiotics is hindered by the harsh conditions of gastrointestinal tract (GIT). Here, we devised a straightforward yet potent modified prebiotic-based "shield" (Gelatin-Inulin, GI), employing dietary inulin and natural polymer gelatin crosslinked via hydrogen bonding for enveloping Lactobacillus reuteri (Lr) to formulate synbiotic hydrogel capsules (Lr@Gl). The GI "shield" serves as a dynamic barrier, augmenting the resistance of Lr to gastric acid and facilitating its bioactivity and adherence in the GIT, synergizing with Lr to elicit an anti-tumor effect. Simultaneously, Lr@GI demonstrates anti-tumor effects by depleting glutathione to release reactive oxygen species, accompanied by the activation of NLRP3 (NOD-like receptor family pyrin domain containing 3), and the induction M1 macrophage polarization. Furthermore, Lr@GI can not only promote the recovery of intestinal barrier but also regulate intestinal flora, promoting the production of SCFAs and further exerting anti-tumor effect. Crucially, Lr@GI also potentiates the anti-tumor effect of 5-Fluorouracil. The construction and synergistic anti-tumor mechanism of synbiotic hydrogel capsules system provide valuable insights for gut microbial tumor therapy.
Keywords: Colon cancer; Lactobacillus reuteri; M1 macrophage; NLRP3; Short-chain fatty acids.
© 2025 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






Similar articles
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
Synbiotics, prebiotics and probiotics for people with chronic kidney disease.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD013631. doi: 10.1002/14651858.CD013631.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870148 Free PMC article.
-
Gut microbiome-based interventions for the management of obesity in children and adolescents aged up to 19 years.Cochrane Database Syst Rev. 2025 Jul 10;7(7):CD015875. doi: 10.1002/14651858.CD015875. Cochrane Database Syst Rev. 2025. PMID: 40637175 Review.
-
Gegen Qinlian decoction ameliorates TNBS-induced ulcerative colitis by regulating Th2/Th1 and Tregs/Th17 cells balance, inhibiting NLRP3 inflammasome activation and reshaping gut microbiota.J Ethnopharmacol. 2024 Jun 28;328:117956. doi: 10.1016/j.jep.2024.117956. Epub 2024 Feb 29. J Ethnopharmacol. 2024. PMID: 38428658
-
A synbiotic combination of mixed probiotics and oligofructose restores intestinal microbiota disturbance in DSS-induced colitis in mice.Front Microbiol. 2025 Jul 24;16:1582155. doi: 10.3389/fmicb.2025.1582155. eCollection 2025. Front Microbiol. 2025. PMID: 40778208 Free PMC article.
References
-
- Janney A., Powrie F., Mann E.H. Host–microbiota maladaptation in colorectal cancer. Nature. 2020;585:509–517. - PubMed
-
- Park E.M., Chelvanambi M., Bhutiani N., Kroemer G., Zitvogel L., Wargo J.A. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28:690–703. - PubMed
-
- Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605–616. - PubMed
LinkOut - more resources
Full Text Sources

