Molecular mechanisms and potential implications of ferroptosis, cuproptosis, and disulfidptosis in septic lung injury
- PMID: 40893878
- PMCID: PMC12394052
- DOI: 10.3389/fmed.2025.1615264
Molecular mechanisms and potential implications of ferroptosis, cuproptosis, and disulfidptosis in septic lung injury
Abstract
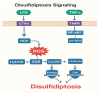
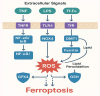
Sepsis remains a life-threatening condition worldwide, causing significant morbidity and mortality across diverse patient populations. Among the various organs adversely affected by sepsis, the lung is particularly vulnerable, often succumbing to acute lung injury (ALI) or its more severe form, acute respiratory distress syndrome (ARDS). Recent basic and translational research has highlighted the importance of multiple regulated cell death (RCD) pathways beyond traditional apoptosis in the pathogenesis of septic lung injury. Three such RCDs, termed ferroptosis, cuproptosis, and disulfidptosis, are increasingly studied for their relevance to critical illnesses. Ferroptosis involves iron-driven lipid peroxidation, cuproptosis depends on copper ion imbalance and mitochondrial protein aggregation, and disulfidptosis emerges from dysregulated sulfide metabolism leading to excessive disulfide bond formation. This review provides an extensive discussion of these RCD pathways within the context of sepsis-induced lung injury. We begin by summarizing the current state of knowledge in septic lung injury, emphasizing inflammatory, immunological, and oxidative stress mechanisms. We then provide a detailed overview of ferroptosis, cuproptosis, and disulfidptosis, illustrating their molecular underpinnings and how they intersect with established sepsis pathways, such as tumor necrosis factor (TNF), nuclear factor kappa B (NF-κB), and mitogen-activated protein kinase (MAPK) signaling cascades. We also discuss emerging findings on the crosstalk among these RCD modes, potential biomarkers for early detection, and therapeutic targets for modulating these pathways. Although many of these findings remain in the early stages of translational research, they collectively underscore the complexity of septic lung injury and offer new directions for improving clinical management. Future investigations, bolstered by integrative "omics" approaches, refined animal models, and well-designed clinical trials, will be pivotal to fully realize the diagnostic and therapeutic potential of ferroptosis, cuproptosis, and disulfidptosis in sepsis. We further propose a "redox stress-metal homeostasis-sulfur metabolism" triangular network, centered on Nrf2's dual regulation of iron/copper transporters and glutathione synthesis, as a unifying framework for RCD modulation in sepsis. A signaling interaction diagram highlights actionable targets for combinatorial therapies.
Keywords: copper homeostasis; cuproptosis; disulfidptosis; ferroptosis; iron metabolism; lung injury; sepsis; sulfide metabolism.
Copyright © 2025 Li, Liu, Shan, Zhong and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Cuproptosis: a novel therapeutic mechanism in lung cancer.Cancer Cell Int. 2025 Jun 24;25(1):231. doi: 10.1186/s12935-025-03864-1. Cancer Cell Int. 2025. PMID: 40555995 Free PMC article. Review.
-
Revolutionizing breast cancer treatment: Harnessing the related mechanisms and drugs for regulated cell death (Review).Int J Oncol. 2024 May;64(5):46. doi: 10.3892/ijo.2024.5634. Epub 2024 Mar 8. Int J Oncol. 2024. PMID: 38456493 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. International forum of acute care, assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. doi: 10.1164/rccm.201504-0781OC - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

