Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): the interplay of gut microbiome, insulin resistance, and diabetes
- PMID: 40893881
- PMCID: PMC12392282
- DOI: 10.3389/fmed.2025.1618275
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): the interplay of gut microbiome, insulin resistance, and diabetes
Abstract
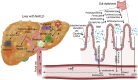
The global prevalence of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) has reached alarming levels, affecting nearly one-third of the world's population. This review analyzes current evidence on the intricate relationships between MASLD, insulin resistance, and type 2 diabetes mellitus (T2DM), with particular emphasis on gut microbiome interactions. As MASLD progresses from simple steatosis to Metabolic Dysfunction-Associated Steatohepatitis (MASH), it can lead to severe complications including fibrosis, cirrhosis, and hepatocellular carcinoma. The pathogenesis of MASLD is multifactorial, involving hepatic lipid accumulation, oxidative stress, inflammation, and dysregulation of the gut-liver axis. Insulin resistance is a central driver of disease progression, closely linked to obesity and metabolic syndrome. Recent research highlights how gut microbiome dysbiosis exacerbates MASLD through mechanisms such as increased intestinal permeability, systemic inflammation, and altered metabolic signaling. Identification of microbial signatures offers promise for novel diagnostic and therapeutic strategies. By integrating metabolic, inflammatory, and microbial perspectives, this review provides a comprehensive overview of MASLD pathogenesis and its association with obesity, insulin resistance, and T2DM.
Keywords: MASH; MASLD; NAFLD; NASH; dysbiosis; metabolic syndrome; microbiome.
Copyright © 2025 Dua, Kumari, Singh, Kumar, Pradeep, Ojesina and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

