This is a preprint.
Mesenchymal Stromal Cell Therapy Restores Intestinal Integrity and Attentuates Inflammation in a Preterm Piglet Model of Necrotizing Enterocolitis
- PMID: 40894018
- PMCID: PMC12393481
- DOI: 10.21203/rs.3.rs-7285196/v1
Mesenchymal Stromal Cell Therapy Restores Intestinal Integrity and Attentuates Inflammation in a Preterm Piglet Model of Necrotizing Enterocolitis
Abstract
Purpose: Necrotizing enterocolitis (NEC) is a life-threatening gastrointestinal disease of prematurity characterized by inflammation, necrosis, and high morbidity. Current therapies are limited, necessitating the development of novel treatments. Mesenchymal stromal cells (MSCs) have shown promise in murine NEC models. Given the anatomical and physiological similarities between premature piglets and human infants, we employed a preterm piglet model to evaluate MSC efficacy. We hypothesized that intraperitoneal MSC administration would reduce intestinal injury in NEC.
Methods: Preterm piglets were delivered via cesarean section. NEC was induced on day 3 through hypertonic enteral feeding. MSCs were administered intraperitoneally at low, medium, or high doses. Piglets were monitored and euthanized based on clinical criteria. Clinical scores, weight change, gross and histologic intestinal injuries were assessed. Cytokine levels in serum and ileum were quantified via ELISA, and intestinal tissue was analyzed by RNA sequencing. Statistical significance was set at p < 0.05.
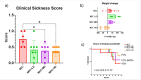
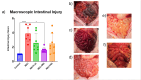
Results: Medium-dose MSCs significantly improved clinical scores and reduced both gross and histologic intestinal injury (p < 0.05). A corresponding decrease in pro-inflammatory cytokines was observed.
Conclusion: This is the first study to demonstrate therapeutic benefit of MSCs in a preterm piglet NEC model, supporting their potential use in translational NEC therapies.
Keywords: Inflammation; Mesenchymal Stromal Cells; Necrotizing Enterocolitis; Preterm Piglet; Stem Cell Therapy.
Conflict of interest statement
Conflicts of Interest / Competing Interests The authors declare no competing interests.
Figures




References
-
- Fitzgibbons SC et al. (2009) Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg, 44(6): pp. 1072–5; discussion 1075–6 - PubMed
-
- Hunter CJ et al. (2008) Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63(2):117–123 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

