High-density lipoproteins. Part 2. Impact of disease states on functionality
- PMID: 40894318
- PMCID: PMC12391827
- DOI: 10.1016/j.ajpc.2025.101073
High-density lipoproteins. Part 2. Impact of disease states on functionality
Abstract
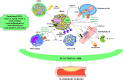
In contrast to low-density lipoproteins which are atherogenic, high-density lipoproteins (HDL) have been conceptualized as beneficial modulators of adverse pathophysiological phenomena along arterial walls. The HDLs are characterized by highly complex and varied molecular cargoes that include apoproteins, enzymes, microRNAs, bioactive lipids and phospholipids, components of complement, and immune factors, among others. These cargo components determine its functionality. Despite the findings of Mendelian inheritance studies which suggest that HDL is not causal in the pathway for atherogenesis, experiments with HDLs show that it can drive reverse cholesterol transport and antagonize inflammation, oxidation, thrombosis, platelet aggregation, endothelial progenitor cell mobilization, potentiate immunity, foster communication between different cell and tissue types, and function as a crucial apoprotein donor amongst the various lipoproteins. These functions are understandably viewed as beneficial and antagonize pathophysiology. Secondary to the complexity of its proteome and lipidome, HDL functionality is profoundly responsive to the metabolic and genetic backgrounds of individuals. Even its size and lipidation status can influence its functionality. As part of the acute phase response, critical antioxidative moieties can be replaced by such acute phase reactants as serum amyloid A and pro-oxidative enzymes. The functionality of HDL is influenced by chronic kidney disease, coronary artery disease, acute myocardial infarction, obesity, insulin resistance, metabolic syndrome, diabetes mellitus, and cancer. Herein we describe many of the alterations in HDL constitution and the resulting changes in functional capacity that can be observed. A unifying theme characterizing these disease states is that they all heighten systemic inflammatory tone and potentiate a pro-oxidative state. These changes clearly associate with profound changes in the functionality and behavior of HDL particles. We are only beginning to comprehend the extraordinary complexity and range of biochemical functions, both beneficial and injurious, that this lipoprotein can regulate. Hence it was extremely premature to think that simply raising HDL cholesterol in serum would beneficially influence cardiovascular morbidity and mortality. We have a long way to go before we develop a more comprehensive and potentially therapeutically relevant understanding of how to better harness its potential for antagonizing disease and block its ability to participate in and adversely influence the course of disease.
Keywords: Atherosclerosis; Epidemiology; High-density lipoprotein; Inflammation; Oxidation; Reverse cholesterol transport; Thrombosis.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
None of the authors have potential conflicts related to the topics covered. None of the authors are on a speakers bureau or a consultant for companies developing HDL therapeutics.
Figures



References
-
- Fielding C.J., Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. - PubMed
-
- Toth P.P., Barter P.J., Rosenson R.S., et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. - PubMed
-
- Ferrara A., Barrett-Connor E., Total Shan J. LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984-1994. Circulation. 1997;96:37–43. - PubMed
-
- Holven K.B., Roeters van Lennep J. Sex differences in lipids: A life course approach. Atherosclerosis. 2023;384 - PubMed
-
- Stamford B.A., Matter S., Fell R.D., Sady S., Papanek P., Cresanta M. Cigarette smoking, exercise and high density lipoprotein cholesterol. Atherosclerosis. 1984;52:73–83. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

