This is a preprint.
Reelin Controls the Directional Orientation, Apical Localization and Length of Primary Cilia in Principal Neurons in the Cerebral Cortex
- PMID: 40894628
- PMCID: PMC12393522
- DOI: 10.1101/2025.08.24.672028
Reelin Controls the Directional Orientation, Apical Localization and Length of Primary Cilia in Principal Neurons in the Cerebral Cortex
Abstract
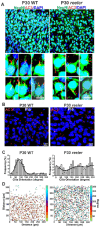
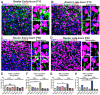
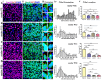
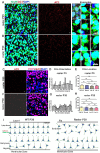
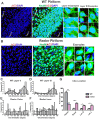
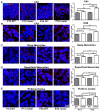
The primary cilia of pyramidal neurons in inside-out laminated regions orient predominantly toward the pia, reflecting reverse soma movement during postnatal neurodevelopment. However, the mechanisms underlying the directional cilia orientation are unknown. Here we show that the primary cilia of pyramidal neurons are localized near the base of the apical dendrites and aligned on the nuclear side opposite to the axon initial segment (AIS). However, this pattern is not observed in atypical pyramidal neurons in the deep neocortex, excitatory neurons in non-laminated regions, interneurons, or astrocytes, where cilia are irregularly positioned around the nuclei and lack preferred orientation. In Reelin-deficient mice (reeler), the directional orientation and apical location of cilia in late-born neocortical and CA1 neurons are disrupted. However, the initial impairments are partially corrected during postnatal development, along with a realignment of apical-basal orientation. In contrast, loss of Reelin drastically disrupts the directional orientation of cilia in early-born neocortical neurons and principal neurons in evolutionarily conserved cortical regions, which lack postnatal correction. Consistently, their cilia do not preferably localize to the apical side. Additionally, Reelin deficiency increases the cilia length of principal neurons across the cerebral cortex at a developmental stage when cilia stabilize in wild-type mice, but this effect is not observed in interneurons, astrocytes, or excitatory neurons in non-laminated regions. Together, Reelin controls the directional orientation, apical localization, and length of primary cilia in principal neurons in the cerebral cortex, underscoring the cilium as a key apical domain particularly prominent in late-born neurons.
Keywords: Apical-Basal Neuron Polarity; Cilia Orientation; Postnatal Neuron Positioning; Primary Cilia; Reelin; Reverse Movement.
Figures



References
-
- Bangs F., and Anderson K.V.. 2017. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb Perspect Biol. 9.
-
- Barbas H. 2015. General cortical and special prefrontal connections: principles from structure to function. Annu Rev Neurosci. 38:269–289. - PubMed
-
- Bishop G.A., Berbari N.F., Lewis J., and Mykytyn K.. 2007. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 505:562–571. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
