Targeting synergetic endothelial inflammation by inhibiting NFKB and JAK-STAT pathways
- PMID: 40894883
- PMCID: PMC12396245
- DOI: 10.1016/j.isci.2025.113307
Targeting synergetic endothelial inflammation by inhibiting NFKB and JAK-STAT pathways
Abstract
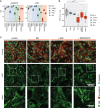
Multiple systemic vascular inflammatory disorders are associated with endothelial dysfunction and elevated levels of TNFα and IFNγ. Combined TNFα and IFNγ stimulation induces synergetic hyperinflammation in endothelial cells (ECs) through the activation of the NFKB and JAK/STAT pathways. Here, we assess how targeting these pathways affects EC inflammation. Using mass spectrometry based proteomics, we investigate system-wide effects of TNFα- and IFNγ-stimulated Endothelial Colony Forming Cells (ECFCs) in combination with inhibitors targeting NFKB and JAK/STAT pathways. JAK1 inhibitor itacitinib blocked IFNγ-, but not TNFα-induced proteomic responses. IKK2/STAT3 inhibitor TPCA1 attenuated both responses. Most TNFα+IFNγ-induced proteins, such as pyroptosis mediators, chemokines, and Weibel-Palade Body content, were inhibited by both inhibitors, highlighting their synergetic dependency on both pathways. Imaging of Von Willebrand Factor (VWF) revealed an extracellular VWF network induced by combined stimulation, a phenotype which was reverted by both inhibitors. This study provides a preliminary basis for inhibiting endothelial inflammation in vascular inflammatory disorders.
Keywords: Biochemistry; Cell biology; Proteomics.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

