Gene expression dynamics before and after zygotic gene activation in Drosophila early embryogenesis
- PMID: 40894916
- PMCID: PMC12396305
- DOI: 10.1016/j.isci.2025.113272
Gene expression dynamics before and after zygotic gene activation in Drosophila early embryogenesis
Abstract
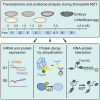
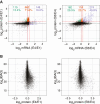
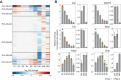
Post-transcriptional gene regulatory mechanisms are fundamental to the determination of gene expression dynamics and especially crucial for the earliest stages of animal development in which transcription is nearly silent. Here, we performed high-resolution total RNA sequencing and quantitative mass spectrometry analysis simultaneously on Drosophila maternal-to-zygotic transition (MZT). Further, this study is the first to report the proteome-wide quantitative changes in protein ubiquitination in Drosophila MZT. Our results indicate that timely ubiquitination of the distinct target proteins during MZT is essential for the downregulation of protein expression levels. Profiling of the RNA-associated proteome changes in Drosophila MZT suggested that RNA binding can be regulated without the respective change in net protein expression levels for over 200 proteins, including Pcid2, Sym, and Cpsf73. We report that highly dynamic and post transcriptionally regulated protein expression level changes can occur at the earliest stages of the Drosophila MZT.
Keywords: Biological sciences; Developmental biology; Natural sciences; Transcriptomics.
© 2025 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

