Role and mechanism of Integrin α5β1 in bone formation and disease
- PMID: 40894924
- PMCID: PMC12391163
- DOI: 10.3389/fcell.2025.1632710
Role and mechanism of Integrin α5β1 in bone formation and disease
Abstract
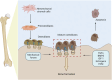
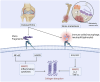
Integrin α5β1 is a key signaling protein between cells and the extracellular matrix. It plays crucial roles in biological processes such as cell adhesion, migration, and differentiation. Recent studies have shown that integrin α5β1 is significantly involved in bone formation and related diseases. Integrin α5β1 participates in the differentiation of mesenchymal stem cells into osteoblasts. It interacts with the CCN family and the bone morphogenetic protein pathway to upregulate the expression of osteogenic markers, promoting the formation of mineralization nodules. Additionally, it can mediate mechanical force stimulation to upregulate osteogenic gene expression and promote bone formation. In diseases such as osteoporosis, osteoarthritis, and bone metastasis, integrin α5β1 mediates abnormal cell-matrix adhesion and migration, promoting pathological bone resorption and inhibiting bone formation, thereby exacerbating bone loss. Therefore, integrin α5β1 may be a potential therapeutic target for these bone diseases. Elucidating its mechanism of action will help understand the homeostatic regulation of bone metabolism and provide ideas for the development of novel therapeutic strategies for skeletal diseases.
Keywords: bone formation; bone metastasis; integrin α5β1; osteoarthritis; osteoporosis.
Copyright © 2025 Li, Hu, Guo, Chang, Yi and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Expression of the integrin alpha5 subunit and its mediated cell adhesion in hepatocellular carcinoma.J Cancer Res Clin Oncol. 1997;123(8):435-40. doi: 10.1007/BF01372547. J Cancer Res Clin Oncol. 1997. PMID: 9292706 Free PMC article.
-
Anterior Approach Total Ankle Arthroplasty with Patient-Specific Cut Guides.JBJS Essent Surg Tech. 2025 Aug 15;15(3):e23.00027. doi: 10.2106/JBJS.ST.23.00027. eCollection 2025 Jul-Sep. JBJS Essent Surg Tech. 2025. PMID: 40821726 Free PMC article.
-
Role of RGD-binding Integrins in ovarian cancer progression, metastasis and response to therapy.Integr Biol (Camb). 2025 Jan 8;17:zyaf003. doi: 10.1093/intbio/zyaf003. Integr Biol (Camb). 2025. PMID: 39916547 Review.
-
Nrf2 signaling pathway: focus on oxidative stress in osteoporosis.Osteoporos Int. 2025 Jul 15. doi: 10.1007/s00198-025-07592-0. Online ahead of print. Osteoporos Int. 2025. PMID: 40663116 Review.
References
-
- Attur M. G., Dave M. N., Clancy R. M., Patel I. R., Abramson S. B., Amin A. R. (2000). Functional genomic analysis in arthritis-affected cartilage: yin-yang regulation of inflammatory mediators by alpha 5 beta 1 and alpha V beta 3 integrins. J. Immunol. 164 (5), 2684–2691. 10.4049/jimmunol.164.5.2684 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

