Stem cell therapy offers new hope for the treatment of Alzheimer's disease
- PMID: 40894925
- PMCID: PMC12391054
- DOI: 10.3389/fcell.2025.1650885
Stem cell therapy offers new hope for the treatment of Alzheimer's disease
Abstract
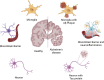
Alzheimer's disease (AD) is a progressive neurodegenerative disorder primarily characterized by memory impairment and cognitive decline, for which no curative treatment is currently available. Existing therapeutic strategies, such as cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists, can only provide limited symptomatic relief and fail to halt disease progression. In recent years, stem cell therapy has emerged as a promising approach for AD due to its multifaceted mechanisms of action. The therapeutic effects of stem cells in AD are mainly attributed to their ability to differentiate into functional neurons or glial cells, thereby replacing damaged cells and repairing neural networks. In addition, stem cells secrete neurotrophic and anti-inflammatory factors that contribute to the improvement of the brain microenvironment. Furthermore, they can regulate neuroinflammation, promote the clearance of β-amyloid (Aβ) deposits, and suppress neuroinflammation, thus potentially slowing disease progression. However, several challenges remain, including low cell survival rates, immune rejection, tumorigenic risks, and difficulties in crossing the blood-brain barrier. Looking ahead, the integration of advanced technologies such as organoid models, gene editing, artificial intelligence, and multi-omics approaches may drive substantial progress in the clinical translation of stem cell therapies for AD. Although still in its early stages, the future of this therapeutic strategy holds great promise.
Keywords: alzheimer’s disease; amyloid-β clearance; paracrine and immunomodulatory mechanisms; regenerative therapy; stem cells.
Copyright © 2025 He, Huang, Zeng, Sun, Wu, Xu, Hu, Jin, Tong and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
miRNAs and Stem Cells as Promising Diagnostic and Therapeutic Targets for Alzheimer's Disease.J Alzheimers Dis. 2023;94(s1):S203-S225. doi: 10.3233/JAD-221298. J Alzheimers Dis. 2023. PMID: 37212107 Free PMC article. Review.
-
Recent advances in the detection and management of motor dysfunction in Alzheimer's disease.Psychiatriki. 2025 Jul 2;36(2):97-100. doi: 10.22365/jpsych.2025.012. Epub 2025 May 14. Psychiatriki. 2025. PMID: 40400272 English, Greek, Modern.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
References
Publication types
LinkOut - more resources
Full Text Sources

