Chimeric anti-HLA antibody receptor engineered human regulatory T cells suppress alloantigen-specific B cells from pre-sensitized transplant recipients
- PMID: 40895551
- PMCID: PMC12394524
- DOI: 10.3389/fimmu.2025.1601385
Chimeric anti-HLA antibody receptor engineered human regulatory T cells suppress alloantigen-specific B cells from pre-sensitized transplant recipients
Abstract
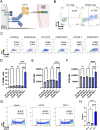
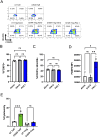
Organ transplantation is a lifesaving procedure, with 50,000 transplants happening every year in the United States. However, many patients harbor antibodies and B cells directed against allogeneic human leukocyte antigen (HLA) molecules, notably HLA-A2, greatly decreasing their likelihood of receiving a compatible organ. Moreover, antibody-mediated rejection is a significant contributor to chronic transplant rejection. Current strategies to desensitize patients non-specifically target circulating antibodies and B cells, resulting in poor efficacy and complications. Regulatory T cells (Tregs) are immune cells dedicated to suppressing specific immune responses by interacting with both innate and adaptive immune cells. Here, we genetically modified human Tregs with a chimeric anti-HLA antibody receptor (CHAR) consisting of an extracellular HLA-A2 protein fused to a CD28-CD3zeta intracellular signaling domain, driving Treg activation upon recognition of anti-HLA-A2 antibodies on the surface of alloreactive B cells. We find that HLA-A2 CHAR Tregs get activated specifically by anti-HLA-A2 antibody-producing cells. Of note, HLA-A2 CHAR activation does not negatively affect Treg stability, as measured by expression of the Treg lineage transcription factors FOXP3 and HELIOS. Interestingly, HLA-A2 CHAR Tregs are not cytotoxic towards anti-HLA-A2 antibody-producing cells, unlike HLA-A2 CHAR modified conventional CD4+ T cells. Importantly, HLA-A2 CHAR Tregs recognize and significantly suppress high affinity IgG antibody production by B cells from HLA-A2 sensitized patients. Altogether, our results provide proof-of-concept of a new strategy to specifically inhibit alloreactive B cells to desensitize transplant recipients.
Keywords: B cells; HLA sensitization; antibody production; engineered immune receptors; human immunology; regulatory T cells; transplant rejection; transplantation.
Copyright © 2025 Valentín-Quiroga, Zarauza-Santoveña, López-Collazo and Ferreira.
Conflict of interest statement
A provisional patent application based on the work reported here has been submitted by JV-Q and LF. LF is an inventor and has received royalties from patents on engineered cell therapies, is a consultant with GuidePoint Global and McKesson, and is the founder and CEO of Torpedo Bio. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Update of
-
Chimeric anti-HLA antibody receptor engineered human regulatory T cells suppress alloantigen-specific B cells from pre-sensitized transplant recipients.bioRxiv [Preprint]. 2025 Apr 1:2025.03.27.645777. doi: 10.1101/2025.03.27.645777. bioRxiv. 2025. Update in: Front Immunol. 2025 Aug 15;16:1601385. doi: 10.3389/fimmu.2025.1601385. PMID: 40236118 Free PMC article. Updated. Preprint.
Similar articles
-
Chimeric anti-HLA antibody receptor engineered human regulatory T cells suppress alloantigen-specific B cells from pre-sensitized transplant recipients.bioRxiv [Preprint]. 2025 Apr 1:2025.03.27.645777. doi: 10.1101/2025.03.27.645777. bioRxiv. 2025. Update in: Front Immunol. 2025 Aug 15;16:1601385. doi: 10.3389/fimmu.2025.1601385. PMID: 40236118 Free PMC article. Updated. Preprint.
-
Chimeric HLA antibody receptor T cells to target HLA-specific B cells in solid organ transplantation.HLA. 2023 Oct;102(4):436-448. doi: 10.1111/tan.15146. Epub 2023 Jun 27. HLA. 2023. PMID: 37370222
-
CXCR5 engineered human and murine Tregs for targeted suppression in secondary and tertiary lymphoid organs.Front Immunol. 2025 Jul 1;16:1513009. doi: 10.3389/fimmu.2025.1513009. eCollection 2025. Front Immunol. 2025. PMID: 40666520 Free PMC article.
-
Immunosuppressive T-cell antibody induction for heart transplant recipients.Cochrane Database Syst Rev. 2013 Dec 2;2013(12):CD008842. doi: 10.1002/14651858.CD008842.pub2. Cochrane Database Syst Rev. 2013. PMID: 24297433 Free PMC article.
-
Do CD4+ Foxp3+ Treg cells correlate with transplant outcomes: a systematic review on recipients of solid organ transplantation.Cell Immunol. 2011;270(1):5-12. doi: 10.1016/j.cellimm.2011.05.006. Epub 2011 May 12. Cell Immunol. 2011. PMID: 21640985
References
-
- World Health Organization (WHO) and Organización Nacional de Trasplantes (ONT) . Global Observatory on Donation and Transplantation, International Report on Organ Donation and Transplantation Activities (2022). Available at: https://www.transplant-observatory.org/
-
- Ruiz R, Kirk AD. Long-Term Toxicity of Immunosuppressive Therapy. In: Busuttil RW, Klintmalm GBG, editors. Transplantation of the Liver. Amsterdam, Netherlands: Elsevier; (2015). p. 1354–63.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

