Nanographene horizons: the emerging role of hexa- peri-hexabenzocoronene in functional material design
- PMID: 40895744
- PMCID: PMC12394830
- DOI: 10.1039/d5ra04623h
Nanographene horizons: the emerging role of hexa- peri-hexabenzocoronene in functional material design
Abstract
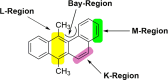
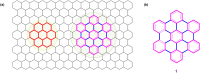
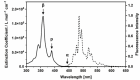
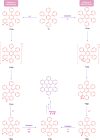
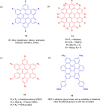
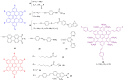
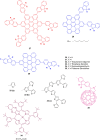
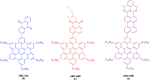
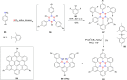
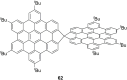
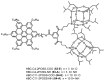
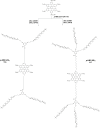
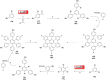
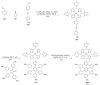
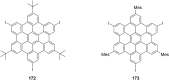
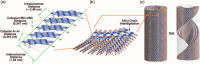
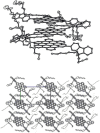
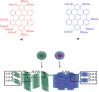
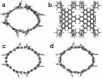
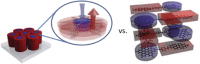
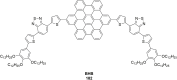
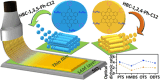
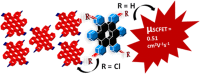
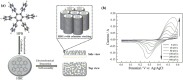
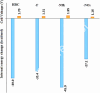
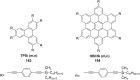
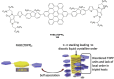
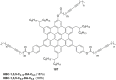
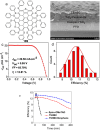
Hexa-peri-hexabenzocoronene (HBC) and its derivatives have emerged as prominent polycyclic aromatic hydrocarbons (PAHs) due to their unique structural, electronic, and photophysical properties. This review provides a comprehensive overview of the synthetic strategies employed for the construction of HBC frameworks, ranging from traditional methods to recent advances that offer improved efficiency, regioselectivity, and structural diversity. The molecular architecture of HBCs, characterized by extended π-conjugation and planarity, contributes significantly to their stability and distinctive physical properties, including high charge-carrier mobility and tunable optical absorption. We further highlight the multifaceted applications of HBC-based materials, particularly in the realm of organic and optoelectronic devices, where their excellent semiconducting behavior and strong π-π stacking facilitate their use in organic field-effect transistors (OFETs), organic photovoltaics (OPVs), and organic light-emitting diodes (OLEDs). Their roles in energy storage, especially in supercapacitors and battery systems, are also discussed, focusing on their ability to enhance charge storage and cycling stability. Moreover, HBCs have demonstrated potential as catalytic platforms and chemical sensors due to their electron-rich surfaces and functionalizable peripheries. Finally, the incorporation of HBC derivatives in biomedical fields such as bioimaging and drug delivery is reviewed, with emphasis on their biocompatibility, fluorescence properties, and structural adaptability. Overall, this article underscores the significant progress in HBC research and its expanding role in diverse scientific and technological domains.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





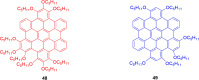







































References
-
- Novoselov K. S. Geim A. K. Morozov S. V. Jiang D. Zhang Y. Dubonos S. V. Grigorieva I. V. Firsov A. A. Science. 2004;306:666–669. - PubMed
-
- Geim A. K. Novoselov K. S. Nat. Mater. 2007;6:183–191. - PubMed
-
- Novoselov K. S. Fal′ko V. I. Colombo L. Gellert P. R. Schwab M. G. Kim K. Nature. 2012;490:192–200. - PubMed
-
- Chen W. Xiao P. Chen H. Zhang H. Zhang Q. Chen Y. Adv. Mater. 2019;31:1802403. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

