Conditioned plasma promotes full-thickness skin defect healing in a rat model
- PMID: 40896180
- PMCID: PMC12396095
- DOI: 10.1016/j.reth.2025.08.003
Conditioned plasma promotes full-thickness skin defect healing in a rat model
Abstract
Introduction: Blood derivatives may enhance wound healing, but each possesses distinct characteristics and has yielded varying outcomes in patient treatment. This research seeks to examine the efficacy of conditioned plasma (CP) using polylactic acid (PLA) coated beads and to compare it with CP using bare beads and platelet-rich plasma (PRP) in the context of acute wound healing.
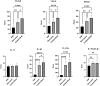
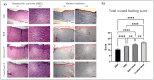
Methods: Blood was collected from 7 volunteer donors in three tubes containing ACD anticoagulant, PLA coated, or bare beads and incubated for 6 h at 37 °C. The concentration of VEGF, PDGF, TGF-β, IL-1β, IL-13, and IL-1Ra were measured by ELISA. Full-thickness wounds were made on the back of rats. PRP, CP with PLA-coated bead or bare beads, and phosphate buffer saline as control were administered to the wound area. Wound closure rate at days 3, 7, 10, and 14; epithelialization, fibroblast cells, inflammatory cells infiltration, new collagen formation, new vessel, and immunohistochemistry (CD31, α-SMA) were measured 14 days after the incision.
Results: The concentration of VEGF, PDGF, TGF-β, IL1-β, and IL-1Ra was significantly higher in CPs than in PRP (p < 0.05). CP with PLA-coated beads promoted wound closure and improved skin wound healing (p < 0.05), which was associated with enhanced epithelialization, fibroblast cell proliferation, new collagen formation, and reduced inflammatory cells infiltration. Immunohistochemistry showed an increase in CD31 and α-SMA levels in the treatment groups compared to the control group, but this increase was insignificant (p > 0.05).
Conclusion: CP promotes wound healing by increasing epithelialization, fibroblast proliferation, collagen synthesis and deposition, and reducing inflammatory cells infiltration.
Keywords: Conditioned plasma; Growth factor; Platelet rich plasma; Regenerative medicine; Wound healing.
© 2025 The Author(s).
Conflict of interest statement
Authors declare no conflict of Interests.
Figures






Similar articles
-
[Experimental study on promotion of skin radiation damage repair by icarin via HIF-2α/VEGF/Notch pathway to enhance the paracrine function of adipose-derived stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025 Jul 15;39(7):881-890. doi: 10.7507/1002-1892.202503089. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025. PMID: 40659593 Free PMC article. Chinese.
-
Dressings and topical agents for the management of open wounds after surgical treatment for sacrococcygeal pilonidal sinus.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013439. doi: 10.1002/14651858.CD013439.pub2. Cochrane Database Syst Rev. 2022. PMID: 35593897 Free PMC article.
-
Poly glycerol sebacate/poly lactide acid (PGS/PLA) hydrogel in combination with hyperbaric oxygen therapy improved full thickness wound healing in diabetic rat.Tissue Cell. 2025 Aug 29;98:103113. doi: 10.1016/j.tice.2025.103113. Online ahead of print. Tissue Cell. 2025. PMID: 40896868
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD009261. doi: 10.1002/14651858.CD009261.pub7. Cochrane Database Syst Rev. 2022. PMID: 35471497 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

