Treatment Regimens with Ranibizumab in Neovascular Age-Related Macular Degeneration: Real-World Results from the PACIFIC Study
- PMID: 40896239
- PMCID: PMC12394746
- DOI: 10.2147/OPTH.S512630
Treatment Regimens with Ranibizumab in Neovascular Age-Related Macular Degeneration: Real-World Results from the PACIFIC Study
Abstract
Introduction: Intravitreal anti-VEGF is the gold standard for treating neovascular age-related macular degeneration (nAMD). The treatment success depends not only on drug efficacy but also on regimen feasibility for physicians and patients. The implementation of different regimen might lead to varying outcomes. "Treat-and-extend" aims to minimize undertreatment with injections at each visit and tailored intervals. This study investigates the utilization and effectiveness of ranibizumab in nAMD, focusing on different treatment regimens in real-world settings.
Materials and methods: The PACIFIC study, a non-interventional, prospective, multicenter study, included nAMD patients treated with ranibizumab at 185 sites across Germany, the Netherlands and Switzerland. Over 24 months, functional and morphological outcomes were documented for 3051 patients over 24 months, highlighting the practiced treatment regimens.
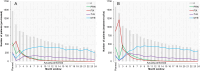
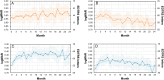
Results: A pattern of an observational approach with nevertheless increasing interval extension prevailed (70.4%, 1028 pre-treated; 68.6%, 1090 treatment-naïve patients), emerging as the preferred strategy within the first 3 months. Across all regimens, the average number of injections was comparable (mean ± SD: 7.34 ± 5.30 in pre-treated; 7.26 ± 4.70 in treatment-naïve patients). The treat and extend regimen, however, demonstrated superior effectiveness in improving visual acuity, particularly among treatment-naïve patients (number of injections: 7.89 ± 5.54 pre-treated; 9.54 ± 5.42 treatment-naïve patients).
Conclusion: Over 2-year observational period, the treat and extend regimen emerged as a highly effective approach, particularly for those newly diagnosed, with a low risk of undertreatment. Despite its benefits, an unconscious shift to a observe-and-extend or "monitor-and-extend" approach occurred early in treatment, highlighting the need for tailored approaches to optimize patient outcomes in clinical practice.
Keywords: anti-VEGF; functional outcome; real-world results; treatment regimen; visual acuity.
© 2025 Lorenz et al.
Conflict of interest statement
Katrin Lorenz receives honoraria from Ethikkommission der Landesärztekammer Rheinland-Pfalz, Novartis Pharma GmbH, and travel grants from Novartis Pharma GmbH, and participated in clinical trials / grants: Aerie, Allergan, Amgen, Bayer, Chengdu Kanghong Biotechnology Co, Hexal, Hoya, iStar, Iveric Bio, Janssen Cilag, Implandata, Lumithera, Microoptx, Mylan, Novartis, Ophtea limited, Pfizer, Redwood, Roche, Sensimed, Santen. Christos Haritoglou receives honoraria as speaker from Novartis and Bayer. Erik Beeke received a grant from Novartis. Matthias Iwersen and Bettina Müller are employees of Novartis Pharma GmbH, Germany. Focke Ziemssen received grants and personal fees from Acelyrin, Alimera, Allergan/Abbvie, Apellis, Bayer Healthcare, BDI, Biogen, Boehringer-Ingelheim, Clearside, CME Health, Ionis, Janssen, Kodiak, Novartis, NovoNordisk, MSD Sharp & Dohme, Oxurion, ODOS, Ophtea, Regeneron, Roche/Genentech, Sandoz, Sanofi, and Stada. The authors report no other conflicts of interest in this work.
Figures
References
-
- Bressler NM. Early detection and treatment of neovascular age-related macular degeneration. J Am Board Fam Pract. 2002;15(2):142–152. - PubMed
-
- Eyetech. MACUGEN (pegaptanib injection) - Highlights of prescribing information. 2011.
LinkOut - more resources
Full Text Sources

