β-Carboline: a privileged scaffold from nature for potential antimalarial activity
- PMID: 40896417
- PMCID: PMC12394914
- DOI: 10.1039/d5md00299k
β-Carboline: a privileged scaffold from nature for potential antimalarial activity
Abstract
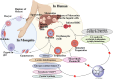
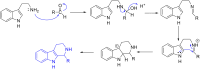
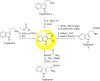
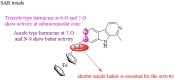
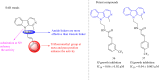
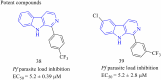
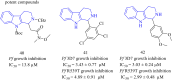
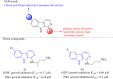
Malaria is one of the most prevalent infectious diseases in the world. Despite the implementation of malaria prophylaxis by the WHO, the mortality rate has been rising. Owing to the development of resistance to presently prescribed antimalarial medication regimes in humans and insecticides in malaria vectors, the prevention and treatment of these illnesses are severely hindered. β-Carbolines are a class of naturally occurring alkaloids that have garnered attention because of their unique structures and diverse biological activities. This review consolidates various methods for synthesizing diverse β-carbolines and highlights their potential as antimalarial agents, emphasizing the molecular targets of Plasmodium falciparum. Based on various research findings, we underscore the potential of β-carbolines to overcome therapeutic challenges and develop effective antimalarials. This review also highlights the structure-activity relationships (SARs) of various β-carboline derivatives to enhance their antimalarial efficacy. With thorough compilation of recent advancements, this review aims to inform and inspire further research and innovation in the quest to combat malaria.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
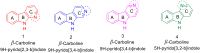

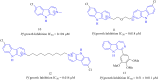
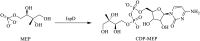

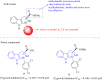
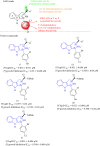



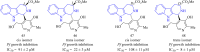
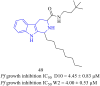


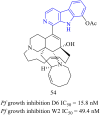
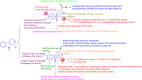
References
-
- Venkatesan P. Lancet Microbe. 2024;5:e214. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

