Two-year outcomes after selective early treatment of patent ductus arteriosus with ibuprofen in preterm babies: follow-up of Baby-OSCAR-a randomised controlled trial
- PMID: 40896454
- PMCID: PMC12396396
- DOI: 10.1016/j.eclinm.2025.103424
Two-year outcomes after selective early treatment of patent ductus arteriosus with ibuprofen in preterm babies: follow-up of Baby-OSCAR-a randomised controlled trial
Abstract
Background: Children born extremely preterm are at increased risk of developmental problems and respiratory morbidity due to patent ductus arteriosus (PDA). The objective of this study was to evaluate whether early treatment of a PDA ≥1.5 mm with ibuprofen improved neurodevelopmental and respiratory outcomes at 24 months of age, corrected for prematurity.
Methods: Baby-OSCAR was a UK multi-center placebo-controlled masked randomized clinical trial in infants born 23+0-28+6 weeks' gestation. The main long-term outcome was survival without moderate or severe neurodevelopmental impairment at 24 months' corrected age, assessed using parent report primarily or classified by blinded end-point review committee where parent-reported data were not available. Other secondary outcomes included survival without respiratory morbidity and duration of oxygen supplementation. (ISRCTN Registry number ISRCTN84264977).
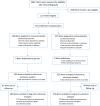
Findings: From July 2015 through December 2020, 653 infants underwent randomization. At 24 months' corrected age, outcome data were available for 537 children: 263 in the ibuprofen group and 274 in the placebo group. Survival without moderate to severe neurodevelopmental impairment in the ibuprofen and placebo groups was 131/248 (53.0%) and 134/259 (51.9%) respectively; adjusted risk ratio 1.01 (95% confidence interval [CI] 0.86-1.18); p = 0.901. Survival without respiratory morbidity was 66/220 (30%) and 74/225 (32.9%) respectively; adjusted risk ratio 0.89 (95% CI 0.68-1.18). Median duration of oxygen supplementation from randomization was 76.0 and 78.0 days, respectively; adjusted median difference -1.5 (-13.8 to 10.9).
Interpretation: We found no evidence of an improvement in neurodevelopmental and respiratory outcomes at 24 months' corrected age, after selective early treatment of a PDA ≥1.5 mm with ibuprofen in children born extremely preterm.
Funding: This study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (11/92/15).
Keywords: Death; Neurodevelopmental outcomes; Patent ductus arteriosus; Premature; Respiratory outcome.
© 2025 The Author(s).
Conflict of interest statement
Samir Gupta received support from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (funding for the Baby-OSCAR study; payments made to institution. Grant Ref: 11/92/15) and has participated as: Member, DSMB, DENSe trial, India; Chair, DMC, TOAST trial (NIHR, UK); Member, DMC, EMBRACE trial, UK; Chair, Trial Steering committee NIHR, UK 152188. Heather O'Connor received support from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (funding for the Baby-OSCAR study; payments made to institution. Grant Ref: 11/92/15). Ed Juszczak received support from the NIHR (Payments were made to my institution). Nimish Subhedar received research grants for neonatal pulmonary hypertension registry from Mallinckrodt Pharmaceuticals; Beyond Air and sponsorship of educational symposium Sept 2023 from Malinckrodt Pharmaceuticals. Pollyanna Hardy received project funding from NIHR HTA (made to institution) and has participated as a committee member on NIHR HTA Commissioning Board. Samantha Johnson received support from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (funding for the Baby-OSCAR study; payments made to institution. Grant Ref: 11/92/15). Ursula Bowler, Charlotte Clarke, David Field, Elizabeth Hutchison, Wilf Kelsall, Justine Pepperell, Tracy Roberts, Sunil Sinha, Kayleigh Stanbury and Jonathan Wyllie declare no competing interests.
Figures

References
-
- Sellmer A., Bjerre J.V., Schmidt M.R., et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed. 2013;98:F505–F510. - PubMed
-
- Rozé J.C., Cambonie G., Le Thuaut A., et al. Effect of early targeted treatment of ductus arteriosus with ibuprofen on survival without cerebral palsy at 2 years in infants with extreme prematurity: a randomized clinical trial. J Pediatr. 2021;233:33–42.e2. - PubMed
LinkOut - more resources
Full Text Sources

