NRF2 Deficiency in Bladder Epithelial Cells Owing to Ubiquitination by N6-Methyladenosine-Modified TRIM21 Induces Oxidative Stress and Inflammation to Aggravate IC/BPS
- PMID: 40896536
- PMCID: PMC12397555
- DOI: 10.2147/JIR.S545880
NRF2 Deficiency in Bladder Epithelial Cells Owing to Ubiquitination by N6-Methyladenosine-Modified TRIM21 Induces Oxidative Stress and Inflammation to Aggravate IC/BPS
Abstract
Background: Interstitial cystitis/bladder pain syndrome (IC/BPS) has become a pressing clinical issue due to its unclear etiology and severe, persistent pelvic pain. Despite extensive research, the pathogenesis of IC/BPS remains unresolved, and current treatments primarily target symptom relief rather than addressing underlying disease mechanisms. This study aimed to investigate the effects of nuclear factor erythroid 2-related factor 2 (NRF2) on IC/BPS and the potential molecular mechanisms.
Methods: Bladder mucosal biopsies from IC/BPS patients were subjected to RT-qPCR and immunoblotting to quantify NRF2 mRNA/protein expression. In vivo modeling, WT and NRF2 gene knockout mice received intraperitoneal cyclophosphamide to induce cystitis. Bladder function was assessed via Void Spot Assays, and Urodynamic. In vitro validation, LPS-stimulated SV-HUC-1 cells were transduced with NRF2 knockdown or overexpression, and oxidative stress and inflammation levels were evaluated. Then, the molecular mechanism of NRF2 in IC/BPS was determined by conducting Western blot, mass spectrometry, co-immunoprecipitation, and RT-qPCR analyses.
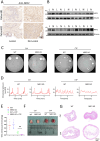
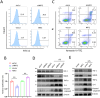
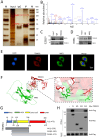
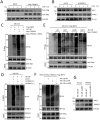
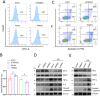
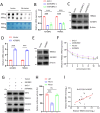
Results: This study identified markedly reduced expression of NRF2 in the lesional bladder mucosa of patients with IC/BPS. By employing NRF2 knockout mice and cellular models of bladder inflammation, the essential role of NRF2 in modulating oxidative stress and inflammation was underscored. Furthermore, tripartite motif-containing 21 (TRIM21) interacted with NRF2, promoting its degradation via ubiquitination in bladder epithelial cell lines, thus elucidating TRIM21's regulatory role in bladder inflammation. Additionally, N6-methyladenosine (M6A) modifications recognized by IGF2BP2 enhanced TRIM21 expression by stabilizing TRIM21 mRNA.
Conclusion: This study positions the TRIM21-NRF2 axis as a key regulator of oxidative stress and inflammation in IC/BPS and suggests it as a promising therapeutic target for future IC/BPS interventions.
Keywords: IC/BPS; N6-methyladenosine; NRF2; TRIM21; ubiquitination.
© 2025 Fan et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures

References
LinkOut - more resources
Full Text Sources

