Cardiovascular adverse events associated with epidermal growth factor receptor tyrosine kinase inhibitors in EGFR- mutated non-small cell lung cancer: systematic review and network meta-analysis
- PMID: 40897431
- PMCID: PMC12402974
- DOI: 10.1136/bmj-2024-082834
Cardiovascular adverse events associated with epidermal growth factor receptor tyrosine kinase inhibitors in EGFR- mutated non-small cell lung cancer: systematic review and network meta-analysis
Abstract
Objective: To evaluate the risk of cardiovascular adverse events associated with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer (NSCLC).
Design: Systematic review and network meta-analysis.
Data sources: PubMed, Embase, Web of Science, Scopus, Cochrane Central Register of Controlled Trials, and three clinical trial registries, from inception to 20 November 2024.
Study selection: Randomised controlled trials comparing EGFR tyrosine kinase inhibitor monotherapy with placebo or other treatments in patients with EGFR-mutated NSCLC.
Data extraction and synthesis: Pairs of reviewers extracted data and assessed risk of bias. A random effects network meta-analysis using a frequentist approach compared adverse drug reactions across different treatments. Certainty of evidence was evaluated using the confidence in network meta-analysis approach. Pairwise meta-analyses were done to compare the three generations of EGFR tyrosine kinase inhibitors.
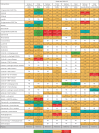
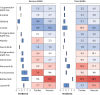
Results: The network meta-analysis comprised 89 randomised controlled trials involving 29 813 participants, mean follow-up 2.18 years. Compared with placebo, both first generation (odds ratio 1.51, 95% confidence interval 1.01 to 2.26; high certainty; pooled incidence 3.2%) and third generation EGFR tyrosine kinase inhibitors (2.18, 1.46 to 3.27; high certainty; 9.5%) were associated with increased risks of cardiac adverse events. Among third generation inhibitors, osimertinib (2.53, 1.53 to 4.19; high certainty; 8.9%) and lazertinib (2.84, 1.17 to 6.91; moderate certainty; 2.7%) were associated with cardiac adverse events. Combined therapies, such as antiangiogenesis with erlotinib or gefitinib, were associated with vascular adverse events (high certainty), whereas antiangiogenesis with osimertinib was associated with cardiovascular adverse events (moderate to high certainty). Also, the risk of arrhythmias was significantly higher with third generation inhibitors (3.26, 1.83 to 5.81; high certainty; 7.3%), such as osimertinib alone (3.35, 1.75 to 6.40; high certainty; 6.2%) and in combination with antiangiogenesis. Almonertinib was associated with vascular toxicity. In pairwise meta-analysis, third generation inhibitors were associated with higher risks of any grade and grade ≥3 cardiovascular adverse events compared with first generation inhibitors.
Conclusions: In patients with EGFR-mutated NSCLC, first and third generation EGFR tyrosine kinase inhibitors, as well as combination therapies with antiangiogenesis, were associated with increased risks of cardiovascular adverse events. Risks were significantly higher with third generation compared with first generation inhibitors.
Systematic review registration: PROSPERO CRD42023433003.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Natural Science Foundation of China; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
