Propofol-based versus sevoflurane-based anaesthesia for deceased donor kidney transplantation: the VAPOR-2 study protocol for an international multicentre randomised controlled trial
- PMID: 40897490
- PMCID: PMC12410677
- DOI: 10.1136/bmjopen-2025-098965
Propofol-based versus sevoflurane-based anaesthesia for deceased donor kidney transplantation: the VAPOR-2 study protocol for an international multicentre randomised controlled trial
Abstract
Introduction: Ischaemia reperfusion injury (IRI) is inevitable in kidney transplantation and negatively affects patient and graft outcomes. Anaesthetic conditioning (AC) refers to the use of anaesthetic agents to mitigate IRI. AC is particularly associated with volatile anaesthetic (VA) agents and to a lesser extent to intravenous agents like propofol. VA like sevoflurane interferes with many of the processes underlying IRI and exerts renal protective properties in various models of injury and inflammation. We hypothesise that a sevoflurane-based anaesthesia is able to induce AC and thereby reduce post-transplant renal injury, reflected in improved graft and patient outcome, compared with a propofol-based anaesthesia in transplant recipients of a deceased donor kidney.
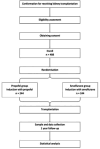
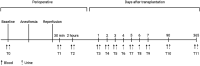
Methods and analysis: Investigator-initiated, multicentre, randomised, controlled and prospective clinical trial with two parallel groups. The study will include 488 kidney transplant recipients from donation after brain death (DBD) or donation after circulatory death (DCD) donors. Participants are randomised in a 1:1 design to a sevoflurane (intervention) or propofol (control) group. The primary endpoint is the incidence of delayed graft function in recipients of DCD and DBD donor kidneys and/or 1-year biopsy-proven and treated acute rejection. Secondary endpoints include functional delayed graft function defined as failure of serum creatinine levels to decrease by at least 10% per day for three consecutive days; primary non-function is defined as a permanent lack of function of the allograft; length of hospital stay and postoperative complications of all kinds, estimated glomerular filtration rate at 1 week and 3 and 12 months calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula; readmissions at 3 and 12 months, graft survival and all-cause mortality at 12 months.
Ethics and dissemination: The study is approved by the local ethical committees and national data security agencies. Results are expected to be published in 2025.
Trial registration number: NCT02727296.
Keywords: ANAESTHETICS; Propofol; Randomized Controlled Trial; Renal transplantation; Transplant medicine; Transplant surgery.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: GJJH has no conflict of interest to report. SPB has received research funding from Novartis and Chiesi. PST has no conflict of interest to report. JH has no conflict of interest to report. DPV has received institutional research grants form Edwards life sciences and Philips Medical BV (not related to this work). LG has no conflict of interest to report. RP has no conflict of interest to report. LLJ has no conflict of interest to report. TIT has no conflict of interest to report. FJB has no conflict of interest to report. CF has no conflict of interest to report. VSG, non-author members, have no conflict of interest to report. KT has no conflict of interest to report. GL has no conflict of interest to report. BJ has no conflict of interest to report. HGDL is Chief Scientific Officer of 34 Lives (West Lafayette, Indiana, USA) and has received institutional research grants from the Dutch Kidney Foundation and NOVO Nordisk Foundation. MMRFS's research group/department received (over the last 3 years) research grants and consultancy fees from Masimo (Irvine, California, USA), Becton Dickinson (Eysins, Switzerland), Fresenius-Kabi (Bad Homburg, Germany), Paion (Aachen, Germany), Medcaptain Europe (Andelst, The Netherlands), Baxter (Chicago, Illinois, USA), HanaPharm (Seoul, Republic of Korea). He receives royalties on intellectual property from Demed Medical (Sinaai, Belgium) and the Ghent University (Gent, Belgium). GJN-M has received research grants from the Dutch Transplant Society, the UMCG Transplant Fund, the European Society of Anaesthesia and Intensive Care, HosmartAI, the De Cock Hadders Foundation and NIAA RCoA/BJA, Sedana Medical and travel grants from the European Society of Anaesthesia and Intensive Care, University Hospital Zurich and the Scandinavia Society of Anaesthesia and Intensive Care; plus, she is an editor of the British Journal of Anaesthesia Education. The funders have no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
