Tissue-specific iron levels modulate lipid peroxidation and the FLASH radiotherapy effect
- PMID: 40897687
- PMCID: PMC12405469
- DOI: 10.1038/s41419-025-07988-0
Tissue-specific iron levels modulate lipid peroxidation and the FLASH radiotherapy effect
Abstract
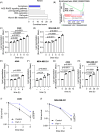
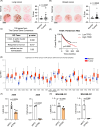
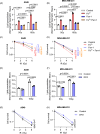
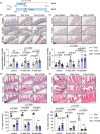
Iron is vital to living cells, playing a key role in cellular respiration, DNA synthesis, and various metabolic functions. Importantly, cancer cells have a higher dependency on iron compared to normal cells to support their rapid growth and survival. Due to this fact, tumors are more vulnerable to ferroptosis, an iron-dependent form of regulated cell death. Radiation therapy (RT), a standard treatment for many cancer patients, is known to induce ferroptosis. Ultra-high dose rate FLASH RT offers an improved therapeutic window by minimizing damage to normal tissues while preserving tumor control. However, the precise biological mechanisms behind the protective effects of FLASH RT on normal tissues remain unclear. In this study, we propose that variations in lipid peroxidation and ferroptosis, driven by intrinsic differences in iron levels between normal and cancerous tissues, contribute to this effect. Our findings show that FLASH RT increases lipid peroxidation and induces ferroptosis in tumor cells but does not significantly elevate lipid peroxidation and ferroptosis in normal tissues compared to conventional RT. To determine whether raising iron levels in normal tissues could abrogate the protective effects of FLASH, mice were fed a high-iron diet before RT. A high-iron diet before and after RT reversed the protective effect of FLASH, resulting in increased intestinal damage and lipid peroxidation. This suggests that baseline iron levels and iron-driven lipid peroxidation are critical factors in mediating the protective outcomes of FLASH RT. Overall, our study sheds light on the role of iron in modulating RT responses and provides new mechanistic insights into how FLASH RT influences normal and cancerous tissues.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Update of
-
Tissue-Specific Iron Levels Modulate Lipid Peroxidation and the FLASH Radiotherapy Effect.bioRxiv [Preprint]. 2025 Jun 11:2025.05.14.653978. doi: 10.1101/2025.05.14.653978. bioRxiv. 2025. Update in: Cell Death Dis. 2025 Sep 2;16(1):668. doi: 10.1038/s41419-025-07988-0. PMID: 40463095 Free PMC article. Updated. Preprint.
References
-
- Federico G, Carrillo F, Dapporto F, Chiariello M, Santoro M, Bellelli R, et al. NCOA4 links iron bioavailability to DNA metabolism. Cell Rep. 2022;40:111207. - PubMed
-
- Hann HW, Stahlhut MW, Menduke H. Iron enhances tumor growth. Observation on spontaneous mammary tumors in mice. Cancer. 1991;68:2407–10. - PubMed
-
- Radulescu S, Brookes MJ, Salgueiro P, Ridgway RA, McGhee E, Anderson K, et al. Luminal iron levels govern intestinal tumorigenesis after Apc loss in vivo. Cell Rep. 2012;2:270–82. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

