Transcriptome dynamics and allele-specific regulation underlie wheat heterosis at the anthesis and grain-filling stages
- PMID: 40898053
- PMCID: PMC12403350
- DOI: 10.1186/s12864-025-11983-2
Transcriptome dynamics and allele-specific regulation underlie wheat heterosis at the anthesis and grain-filling stages
Abstract
Background: As wheat is a globally important staple crop, the molecular regulatory network underlying heterosis in wheat remains incompletely understood. The flag leaf is the primary source of photoassimilates during grain filling and plays a crucial role in yield formation. However, the genetic mechanisms linking flag leaf development to heterosis are still unclear.
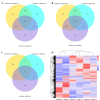
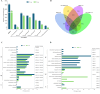
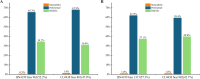
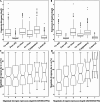
Results: Transcriptomic analysis revealed dynamic transcriptional reprogramming during the anthesis to grain-filling transition, with a pronounced expression bias toward superior parental alleles in hybrids. Anthesis-stage non-additive dominance and grain-filling-stage additive enhancement synergistically orchestrated the temporal regulatory shift underlying heterosis. The dominant alleles from the superior parent accounted for more than 60% of the non-additive genes, and the superior parent bias in allele-specific expression ratios progressively increased during development. This highlighted the role of the superior parent as an allelic reservoir. Cis-regulatory variations primarily contributed to additive effects, whereas cis×trans interactions were the primary regulatory driver of positive overdominance. Notably, weighted co-expression network analysis identified HSP90.2-B as a putative heterosis-related gene, whose coordinated overexpression with AP2/ERF transcription factors provides valuable insights for elucidating the molecular basis of yield heterosis.
Conclusions: This study establishes two complementary models to decode the molecular regulation of heterosis in wheat. The "dual-engine" model demonstrates stage-specific gene expression patterns: non-additive effects predominantly drive early growth vigor during the anthesis stage, whereas additive expression patterns stabilize grain development and yield-related traits at the grain-filling stage. The "two-phase regulatory shift" model captures the dynamic temporal progression of heterotic regulation, evolving from trans-regulation-driven plastic responses at the anthesis stage to cis-regulation-mediated homeostatic control at the grain-filling stage. Importantly, the preferential coupling between cis-regulation/additive and trans-regulation/non-additive expression provides molecular evidence supporting the complementary nature of the models. We further identified developmentally specific modules (the anthesis-stage Red module and grain-filling-stage Brown module) with their core regulatory networks through weighted gene co-expression network analysis. These findings preliminarily characterize the multi-layered cooperative networks regulating heterosis development, potentially offering valuable theoretical clues for deciphering the molecular mechanisms underlying wheat heterosis.
Keywords: Cis- and trans-regulation; HSP90.2-B; Triticum aestivum L.; Additive and non-additive expression patterns; Allele-specific expression; Heterosis; Transcriptome; Weighted gene co-expression network analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Liu W, Zhang Y, He H, He G, Deng X. From hybrid genomes to heterotic trait output: challenges and opportunities. Curr Opin Plant Biol. 2022;66:102193. - PubMed
-
- Duvick DN. Biotechnology in the 1930s: the development of hybrid maize. Nat Rev Genet. 2001;2(1):69–74. - PubMed
-
- Longin CFH, Mühleisen J, Maurer HP, Zhang H, Gowda M, Reif JC. Hybrid breeding in autogamous cereals. Theor Appl Genet. 2012;125(6):1087–96. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

