Automated quantification of abdominal aortic calcification using 3D nnU-Net: a novel approach to assess AAA rupture risk
- PMID: 40898112
- PMCID: PMC12403428
- DOI: 10.1186/s12880-025-01911-x
Automated quantification of abdominal aortic calcification using 3D nnU-Net: a novel approach to assess AAA rupture risk
Abstract
Background: Abdominal aortic aneurysms (AAA) pose a serious rupture risk, heightened by aortic calcification. Traditional calcification scoring methods are slow and require expertise. This study aims to construct a convolutional neural network (nnU-Net) model for automatic quantification and segmentation of abdominal aortic calcification from a single CTA scan.
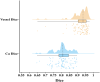
Methods: This retrospective study included 100 patients who underwent abdominal aortic CTA between January 2018 and October 2023, meeting specific inclusion criteria. Vessel and calcification segmentation were manually scored by two physicians, and an nnU-Net deep learning model was developed to automate calcification measurement. Model performance was assessed using Dice scores. Agreement between manual and model-based scoring was assessed using Spearman rank correlation and Bland-Altman analysis.
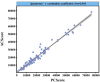
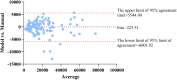
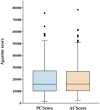
Results: The nnU-Net model achieved median Dice scores of 93.60% for blood vessels and 81.06% for calcification. Average Dice scores were 92.37 ± 4.87% for blood vessel segmentation and 81.03 ± 5.11% for calcified plaque. The model's Agatston scores correlated closely with manual scores (Spearman's ρ = 0.969), with a mean difference of -229.51 (95% limits of agreement: -6003.92 to 5544.90). The model's evaluation time was also shorter than manual scoring (112 ± 4.4 s vs. 3796 ± 6.6 s, p < 0.001).
Conclusion: The nnU-Net-based model shows potential as an automated tool for accurately segmenting and quantifying abdominal aortic calcification, offering comparable results to manual scoring with significantly reduced evaluation time. This approach may assist in more efficient assessment of AAA rupture risk, supporting clinical decision-making in patient management.
Keywords: Abdominal aortic aneurysm; Calcification; Convolutional neural network (nnU-Net); Rupture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Nanfang Hospital (approval number: NFEC-202504-K3-01). Written informed consent was obtained from all individual participants included in this study. All procedures complied with the Declaration of Helsinki. Consent for publication: The dataset used in this study was fully anonymized and complied with ethical standards for retrospective analysis. No direct patient identifiers or sensitive clinical details were included in the manuscript. Data sharing statement: Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available. Competing interests: The authors declare no competing interests.
Figures




References
-
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, Augoustides JG, Beck AW, Bolen MA, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: A report of the American heart association/american college of cardiology joint committee on clinical practice guidelines. Circulation. 2022;146(24):e334–482. - PMC - PubMed
-
- Wanhainen A. Response to re ‘european society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal Aorto-Iliac artery aneurysms’. Eur J Vasc Endovasc Surg. 2020;60(6):951. - PubMed
-
- Vorp DA, Vande Geest JP. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler Thromb Vasc Biol. 2005;25(8):1558–66. - PubMed
-
- Buijs RV, Willems TP, Tio RA, Boersma HH, Tielliu IF, Slart RH, et al. Current state of experimental imaging modalities for risk assessment of abdominal aortic aneurysm. J Vasc Surg. 2013;57(3):851–9. - PubMed
-
- Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(2):92–102. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

