Succinic Acid-Induced Macrophage Endocytosis Promotes Extracellular Vesicle-Based Integrin Beta1 Transfer Accelerating Fibroblast Activation and Sepsis-Associated Pulmonary Fibrosis
- PMID: 40898357
- PMCID: PMC12631846
- DOI: 10.1002/advs.202507411
Succinic Acid-Induced Macrophage Endocytosis Promotes Extracellular Vesicle-Based Integrin Beta1 Transfer Accelerating Fibroblast Activation and Sepsis-Associated Pulmonary Fibrosis
Abstract
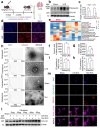
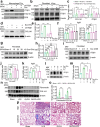
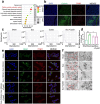
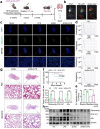
Sepsis-associated pulmonary fibrosis (SAPF) is a life-threatening condition driven by persistent fibroblast activation and excessive extracellular matrix (ECM) deposition. While metabolic reprogramming, profibrotic extracellular vesicles (EVs), and integrin activation are implicated in pulmonary fibrosis, their interplay remains unclear. This study reveals that succinic acid, a product of glycometabolic reprogramming, promotes macrophage-mediated endocytosis, driving the release of profibrotic EVs. These EVs transfer integrin beta1 (ITGβ1) from macrophages to fibroblasts, activating fibroblasts and advancing SAPF. Through Single-cell RNA sequencing (scRNA-seq), proteomics, immunofluorescence, and electron microscopy, the critical role of EV-mediated ITGβ1 transfer in macrophage-fibroblast communication is identified. Knockdown of ITGβ1 or Alix, a mediator of multivesicular bodies (MVBs) biogenesis, inhibited profibrotic EVs formation and alleviated SAPF. These findings highlight a novel mechanism in that the transfer ITGβ1 via EVs plays a critical role in macrophage-fibroblast communication, representing a novel mechanism underlying SAPF. Targeting EV-mediated ITGβ1 transfer can provide a promising therapeutic strategy to alleviate the progression of SAPF.
Keywords: extracellular vesicles; integrin β1; macrophage‐fibroblast communication; pulmonary fibrosis; sepsis.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hotchkiss R. S., Moldawer L. L., Opal S. M., Reinhart K., Turnbull I. R., Vincent J.‐L., Nat. Rev. Dis. Primer 2016, 2, 16045.
-
- Mirzapoiazova T., Kolosova I. A., Moreno L., Sammani S., Garcia J. G. N., Verin A. D., Eur. Respir. J. 2007, 30, 429. - PubMed
-
- Li B., Xia C., He W., Liu J., Duan R., Ji Z., Pan X., Zhou Y., Yu G., Wang L., Adv. Sci. 2024, 11, 2401931.
-
- Xian H., Liu Y., Rundberg Nilsson A., Gatchalian R., Crother T. R., Tourtellotte W. G., Zhang Y., Aleman‐Muench G. R., Lewis G., Chen W., Kang S., Luevanos M., Trudler D., Lipton S. A., Soroosh P., Teijaro J., De La Torre J. C., Arditi M., Karin M., Sanchez‐Lopez E., Immunity 2021, 54, 1463. - PMC - PubMed
-
- Sun Z.‐C., Liao R., Xian C., Lin R., Wang L., Fang Y., Zhang Z., Liu Y., Wu J., J. Controlled Release 2024, 375, 300.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Medical
Miscellaneous
