Formosulfathiazole: A Structural Revision
- PMID: 40899114
- PMCID: PMC12605753
- DOI: 10.1002/cplu.202500406
Formosulfathiazole: A Structural Revision
Abstract
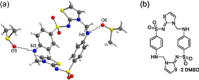
Formosulfathiazole (FSTz) is a synthetic active pharmaceutical ingredient (API) prepared by condensation of sulfathiazole with formaldehyde. Originally described for the first time in 1948, it is currently used for the treatment of bacterial and protozoal infections in cattle and pets, acting as a prodrug slowly releasing the sulfamidic sulfathiazole and formaldehyde. A systematic analysis of FSTz allowed to revise the originally believed undefined polymeric structure and uncovered the intriguing cyclophane skeleton of a well-defined cyclodimeric condensation product.
Keywords: cyclodimer; cyclophane; electron diffraction; formosulfathiazole; structural revision.
© 2025 The Author(s). ChemPlusChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

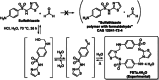
References
-
- Martindale: The Complete Drug Reference (Ed.Brayfield A.), Pharmaceutical Press, London: 2014. - PubMed
-
- Williams M., Drug Dev. Res. 2013, 74, 339.
-
- Brunton L., Knollmann B., Goodman and Gilman's the Pharmacological Basis of Therapeutics, 14th ed., McGraw Hill/Medical, New York: 2022.
-
- Bhatnagar S. S., Fernandes F., Nature 1949, 164, 272. - PubMed
-
- Riviere J. E., Papich M. G., Veterinary Pharmacology and Therapeutics, Blackwell Pub, Hoboken, NJ: 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

