Association of Pharmacologic Markers of Anti-Tumor Necrosis Factor-α Activity and Etanercept Effectiveness in Juvenile Idiopathic Arthritis
- PMID: 40899440
- PMCID: PMC12406077
- DOI: 10.1002/prp2.70166
Association of Pharmacologic Markers of Anti-Tumor Necrosis Factor-α Activity and Etanercept Effectiveness in Juvenile Idiopathic Arthritis
Abstract
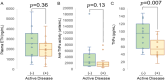
Therapies targeting tumor necrosis factor-α (TNFα), including etanercept (ETN), have become a mainstay in the treatment of juvenile idiopathic arthritis (JIA). As a result, this work seeks to evaluate the relationship between pharmacologic markers of ETN activity and clinical effectiveness in a cohort of patients with JIA. This is a single-centered, open-label, prospective, cross-sectional study of patients with JIA (n = 26) receiving maintenance ETN. Determination of ETN effectiveness was based on the absence of active arthritis in any joint at the time of sample collection. Patient plasma samples were collected and analyzed for ETN concentrations, anti-ETN antibodies, anti-TNFα activity, and TNFα concentrations. ETN was effective in 46% (n = 12) of patients assessed at the time of sampling. No differences in baseline demographics were observed between patients based on effectiveness. Median [IQR] plasma ETN concentrations were 2094 [1384, 2680] ng/mL. Anti-ETN antibodies were undetectable in all patients. Plasma anti-TNFα activity varied 32-fold and was directly proportionate to plasma ETN concentrations (ρ = 0.75, p = 3.8 × 10-3). Plasma TNFα concentrations were 9-fold higher than previous measurements in patients with JIA not receiving anti-TNFα therapy (p = 3.6 × 10-9). ETN effectiveness was associated with 21% higher ETN concentrations (p = 0.36), 52% higher plasma anti-TNFα activity (p = 0.14), and 84% higher plasma TNFα concentrations (p = 0.008). Among pharmacologic markers of ETN activity measured, plasma TNFα concentrations are most strongly associated with ETN effectiveness in JIA.
Keywords: etanercept; juvenile idiopathic arthritis; pharmacokinetics; tumor necrosis factorbiomarkers.
© 2025 The Author(s). Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
R.S.F. is currently an employee of Amgen Inc. This work was completed during his time at The University of Kansas and reflects his views as a scientist, and not those of the company.
Figures



References
-
- Helmick C. G., Felson D. T., Lawrence R. C., et al., “Estimates of the Prevalence of Arthritis and Other Rheumatic Conditions in the United States. Part I,” Arthritis & Rheumatism 58 (2008): 15–25. - PubMed
-
- Becker M. L., “Optimization of Pediatric Rheumatology Therapeutics,” Clinical Pharmacology and Therapeutics 91 (2012): 597–606. - PubMed
-
- Ravelli A., Consolaro A., Horneff G., et al., “Treating Juvenile Idiopathic Arthritis to Target: Recommendations of an International Task Force,” Annals of the Rheumatic Diseases 77 (2018): 819–828. - PubMed
-
- Marotte H. and Cimaz R., “Etanercept—TNF Receptor and IgG1 Fc Fusion Protein: Is It Different From Other TNF Blockers?,” Expert Opinion on Biological Therapy 14 (2014): 569–572. - PubMed
-
- Funk R. S., Chan M. A., and Becker M. L., “Cytokine Biomarkers of Disease Activity and Therapeutic Response After Initiating Methotrexate Therapy in Patients With Juvenile Idiopathic Arthritis,” Pharmacotherapy 37 (2017): 700–711. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

